- Department of Neurosurgery, International University of Health and Welfare, Narita City, Japan.
Correspondence Address:
Keisuke Onoda, Department of Neurosurgery, International University of Health and Welfare, Narita City, Japan.
DOI:10.25259/SNI_748_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Keisuke Onoda, Ryohei Sashida, Yu Hirokawa, Ren Fujiwara, Tomihiro Wakamiya, Yuhei Michiwaki, Tatsuya Tanaka, Kazuaki Shimoji, Eiichi Suehiro, Fumitaka Yamane, Masatou Kawashima, Akira Matsuno. The development of a new, ultra-fine, and flexible neuroendoscope for intracranial observation. 07-Oct-2022;13:460
How to cite this URL: Keisuke Onoda, Ryohei Sashida, Yu Hirokawa, Ren Fujiwara, Tomihiro Wakamiya, Yuhei Michiwaki, Tatsuya Tanaka, Kazuaki Shimoji, Eiichi Suehiro, Fumitaka Yamane, Masatou Kawashima, Akira Matsuno. The development of a new, ultra-fine, and flexible neuroendoscope for intracranial observation. 07-Oct-2022;13:460. Available from: https://surgicalneurologyint.com/surgicalint-articles/11918/
Abstract
Background: A neuroendoscope is a technical advance that allows surgeons to visualize certain regions of the brain that was previously inaccessible through the use of a surgical microscope. Several neuroendoscope designs have been implemented by other neurosurgeons over the past 5 years. The advantage of a neuroendoscope is the addition of a flexible and narrow tip that allows for safe entry into intracranial structures for clinical observation. However, there are some limitations to this approach. Here, we report the use of a modified angioscope as a newly developed neuroendoscope to be employed in observing intracranial structures.
Methods: We report the use of an angioscope that is 1.8 mm in diameter and has both a thin and flexible tip. In this study, the angioscope was inserted into the lumen of an aspirator tube, and the tip of the device was placed at the intracranial area of intended observation area. Image findings were evaluated using an established in vivo goat brain model.
Results: The angioscope was light in weight and maneuverable and could be reached and observed in the blind spot using a surgical microscope. From the cerebellopontine angle, the lower cranial nerves and trigeminal nerve could be observed, and from the cisterna magna, the floor of the fourth ventricle and the aqueduct could be seen.
Conclusion: The angioscope is a useful instrument to observe intracranial locations safely and effectively even within a limited surgical field. Further modifications will be required to use the angioscope in various craniotomy procedures.
Keywords: Angioscope, Intracranial, Neuroendoscope, Neurosurgery
INTRODUCTION
When patients suffer from aneurysm, a procedure known as cerebral aneurysm neck clipping must be performed to prevent ischemia. At present, neurosurgeons must perform microsurgical clipping, where a small metal clip is used to obstruct the ruptured aneurysm and to prevent rebleeding.[
An angioscope is utilized to observe and evaluate the degree of atherosclerosis within the lumen of blood vessels.[
MATERIALS AND METHODS
Animal models
In vivo goat brain model that we recently reported was employed to the present study. Three male goats, which weighed 17.5, 26.5, and 27.5 kg, were considered. We ensured that the study was performed under the conformity of the international, national, and institutional rules considering animal experiments, clinical studies, and biodiversity rights. All manipulations and protocols were performed according to the Guidelines for Animal Experiments at the International University of Health and Welfare and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications no. 85–23) revised in 1996.
Surgery
Animals were initially anesthetized with xylazine 0.4 mg/0.02/kg and atropine 0.5 mg injected intramuscularly, and maintained on 5% isoflurane and 95% O2 at 1.5–2.0 ml/min to control breathing. Anesthesia was maintained with 2% isoflurane and 100% O2 at 1.5–2.0 ml/min.[
In vivo recordings
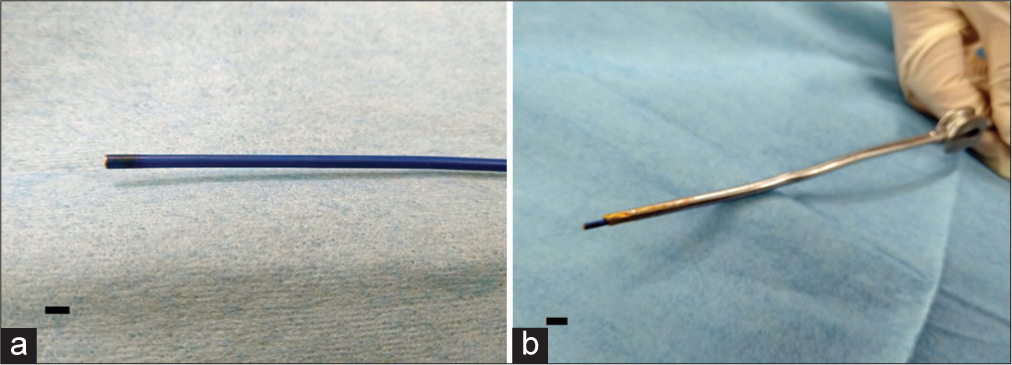
The angioscope used in this study was a Zemporshe videovascular endoscopic catheter with an outer diameter of 1.83 mm, length of 180 cm, a focal range of 2–3 mm, a viewing angle of 92.6°, and a pixel count of 48000 (OVALIS, Osaka, Japan). The angioscope was inserted into a suction tube (Fujita Medical Instruments Co., Ltd Tokyo, Japan) with an inner diameter of 2.5 mm to guide it safely to the deep and narrow areas of the brain.
RESULTS
The angioscope is extremely light in weight, easy to handle, and allows rapid access to the area under observation [
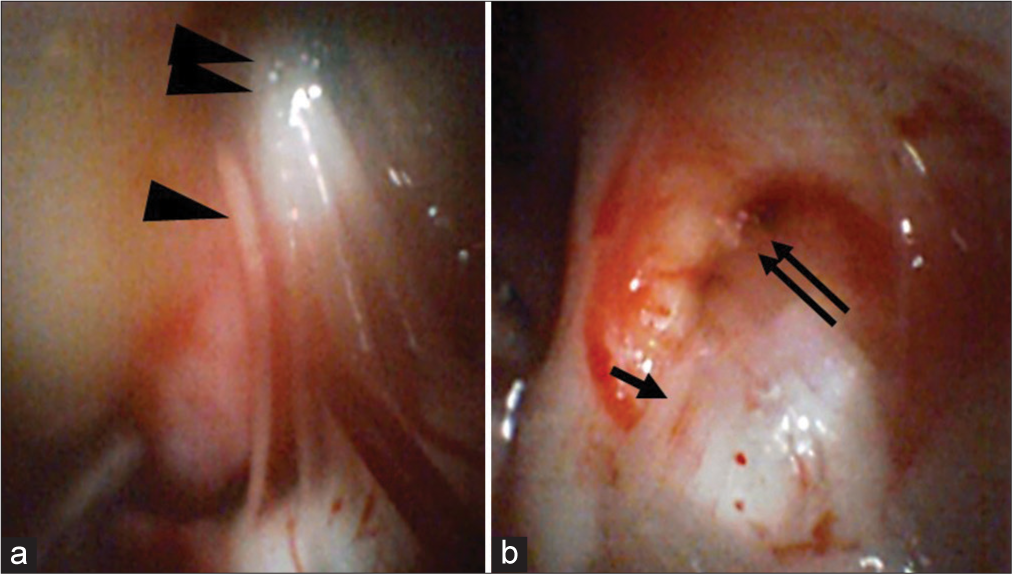
Figure 2:
Observations of an angioscope inserted into the cerebellopontine angle Panel (a) depicts the use of the angioscope to observe the glossopharyngeal (arrow head) and vagus nerves (double arrow heads) in the cerebellopontine angle. Panel (b) indicates the use of the angioscope to observe the trigeminal nerve (arrow) and Meckel’s cavity (double arrows).
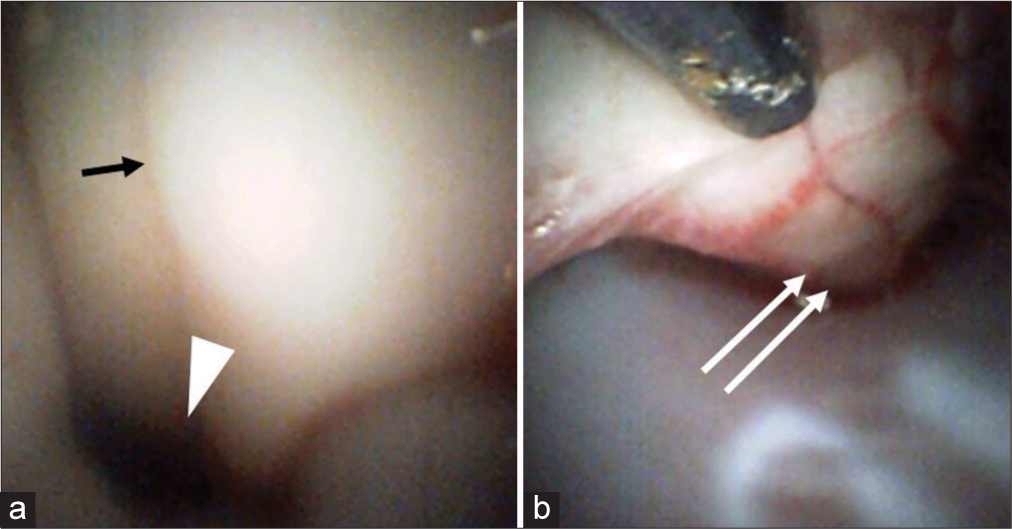
Figure 3:
Observations of an angioscope inserted into the cisterna magna Panel (a) depicts the use of the angioscope to observe the floor of the fourth ventricle of the central sulcus (arrow), as well as the cerebral aqueduct (arrow head). Panel (b) indicates the use of the angioscope inserted through the foramen magnum to observe the anterior surface of the spinal cord (double arrow), which could be evaluated up to the C3 vertebrae.
DISCUSSION
The angioscope is primarily used for observations of the coronary artery. Normal intima of the endothelium is white and smooth, but the lipid deposition provides a yellowish tinting of the endothelial cell wall, allowing angioscope to detect early atherosclerotic lesions that do not cause stenosis.[
Neuroendoscopes are usually used to observe pituitary tumors,[
In this study, a thin, light, and flexible angioscope was inserted into the suction in the present study. The angioscope can be used in the same motion as a suction manipulation, because no particular skill is required to handle it. In addition, double-tube suction may be useful in the fact that it does not interfere with the suction function. We are currently preparing a study of angioscope as neuroendoscope with double-tube suction. The major difference with existing neuroendoscope is that the angioscope can be manipulated in fine movements to intend to observe the optimum site and does not interfere with the surgical manipulations in the limited narrow surgical field because of its thinness. Further refinement of the angioscope for use as a new neuroendoscope has the potential to provide a significant benefit in craniotomy surgery.
There have been no reports of this neurosurgical technique training using live goat brains. We recently reported the usefulness of in vivo goat brain model in neurosurgical training.[
CONCLUSION
The angioscope could be very useful as a neuroendoscope to safely and simply observe any site in craniotomy that cannot be reached by the field of view of a surgical microscope. The angioscope is expected to be developed and refined as a new neuroendoscope through further improvements based on the accumulation of more cases.
Ethical approval
All procedures used in this research were approved by the Ethical Committee of International University of Health and Welfare.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to express our gratitude to OVARIS K.K., led by Mr. Hori and Integra Japan K.K., led by Mr. Ito and Mr. Shibakawa, for their cooperation with goat brain model.
References
1. Almeida JP, de Albuquerque LA, Fabbro MD, Sampaio M, Medina R, Chacon M. Endoscopic skull base surgery: Evaluation of current clinical outcomes. J Neurosurg Sci. 2019. 63: 88-95
2. Azab WA, Abdelrahman AY, Alsheikh TM, Najibullah M. Neuroendoscopy in Kuwait: Evolution, current status and future directions. World Neurosurg. 2016. 92: 298-302
3. Fischer G, Oertel J, Perneczky A. Endoscopy in aneurysm surgery. Neurosurgery. 2012. 70: 184-91
4. Hatada A, Takagaki Y, Yamamoto S, Okamura Y, Nishimura Y. High-resolution angioscopic observation of deep vein thrombosis after catheter-directed venous thrombolysis. Circ J. 2020. 84: 1885
5. Ho CL, Peter Y Hwang PY. Endoscope-assisted transorbital keyhole surgical approach to ruptured supratentorial aneurysms. J Neurol Surg A Cent Eur Neurosurg. 2015. 76: 376-83
6. Ihara M, Nojima Y, Koh N, Adachi H, Kurimoto T, Okayama K. Angioscopic findings of neovascularization around yellow plaque. Circ J. 2021. 85: 1400
7. Ikeoka K, Okayama K, Watanabe T, Nanto S, Sakata Y, Hoshida S. Refractory vascular wall healing after paclitaxel-coated nitinol stent implantation in the femoropopliteal artery: A high-resolution angioscopic assessment. Ann Vasc Dis. 2018. 11: 373-6
8. Kher Y, Yadav N, Yadav YR, Parihar V, Ratre S, Bajaj J. Endoscopic vascular decompression in trigeminal neuralgia. Turk Neurosurg. 2017. 27: 998-1006
9. Lee HS, Kim M, Park JC, Ahn JS, Lee S, Park W. Clinical features of ischemic complications after unruptured middle cerebral artery aneurysm clipping: Patients and radiologically related factors. Neurosurg Rev. 2021. 44: 2819-29
10. Matsukawa H, Kamiyama H, Miyazaki T, Kinoshita Y, Ota N, Noda K. surgical treatment of middle cerebral artery aneurysms: Aneurysm location and size ratio as risk factors for neurologic worsening and ischemic complications. World Neurosurg. 2018. 117: e563-70
11. Onoda K, Fujiwara R, Sashida R, Hirokawa Y, Wakamiya T, Michiwaki Y. In vivo goat brain model for neurosurgical training. Surg Neurol Int. 2022. 13: 344
12. Rocque BG. Neuroendoscopy for intraventricular tumor resection. World Neurosurg. 2016. 90: 619-20
13. Rutkowski M, Zada G. Management of pituitary adenomas invading the cavernous sinus. Neurosurg Clin N Am. 2019. 30: 445-55
14. Tsujimura T, Ishihara T, Iida O, Hata Y, Toyoshima T, Higashino N. Arterial healing 10 months after implantation of an ultrathin-strut, biodegradable-polymer, sirolimus-eluting stent an angioscopic study. Circ Rep. 2021. 3: 316-23
15. Wang P, Li Q, Wang C, Li C. Complete neuroendoscopic versus microscopical trigeminal neuralgia microvascular decompression (MVD) in primary trigeminal neuralgia (PTN). Am J Transl Res. 2021. 13: 12905-12