- Department of Neurosurgery, Medical Faculty, Mataram University, General Province West Nusa Tenggara Hospitals, Mataram, Indonesia.
- Department of Neurosurgery, Udayana University Hospital, Medical Faculty of Udayana University, Bali, Indonesia
- Department of Neurosurgery, Faculty of Medicine, Mataram University, General Province West Nusa Tenggara Hospitals, Mataram, Indonesia.
- Department of Anatomy, Medical Faculty, Mataram University, General Province West Nusa Tenggara Hospitals, Mataram, Indonesia.
- Department of Pathology Anatomy, Medical Faculty, Mataram University, General Province West Nusa Tenggara Hospitals, Mataram, Indonesia.
- Research Unit, Faculty of Medicine, Udayana University, Bali, Indonesia
- Research Unit, Faculty of Medicine, Al Azhar Islamic University, Mataram, Indonesia
Correspondence Address:
Rohadi Muhammad Rosyidi, Department of Neurosurgery, Medical Faculty, Mataram University, General Province West Nusa Tenggara Hospitals, Mataram, Indonesia.
DOI:10.25259/SNI_170_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rohadi Muhammad Rosyidi1, Dewa Putu Wisnu Wardhana2, Bambang Priyanto1, Januarman Januarman3, Decky Aditya Zulkarnaen4, Lale Maulin Prihatina5, Hanan Anwar Rusidi6, Rozikin Rozikin7. The effect of Centella asiatica, cinnamon, and spirulina as neuroprotective based on histopathological findings in ratus Sprague Dawley with traumatic brain injury. 28-Jun-2024;15:217
How to cite this URL: Rohadi Muhammad Rosyidi1, Dewa Putu Wisnu Wardhana2, Bambang Priyanto1, Januarman Januarman3, Decky Aditya Zulkarnaen4, Lale Maulin Prihatina5, Hanan Anwar Rusidi6, Rozikin Rozikin7. The effect of Centella asiatica, cinnamon, and spirulina as neuroprotective based on histopathological findings in ratus Sprague Dawley with traumatic brain injury. 28-Jun-2024;15:217. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12971
Abstract
Background: Traumatic brain injury (TBI) is a global health problem with the potential to cause dangerous neurological problems. Based on histopathological findings in Sprague Dawley (SD) rats with TBI in the acute phase, the study seeks to discover the effect of Centella asiatica, cinnamon, and spirulina as neuroprotective.
Methods: We conducted an experimental study with 30 SD rats randomly divided into three groups. The intervention was the administration of C. asiatica, cinnamon, and spirulina to the control and the experimental groups. Histological features were assessed using hematoxylin and eosin (H&E) staining and immunohistochemical examination. The data were analyzed using statistical analysis through correlation tests.
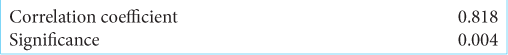
Results: The test samples’ average body weights had P > 0.05, indicating no significant difference in the test sample body weights. Therefore, the variations in the expression level of the dependent variable were expected to be caused by the induction of brain injury and the administration of C. asiatica, cinnamon, and spirulina. In addition, the variables were not normally distributed. Thus, the Spearman test was carried out and showed the correlation was very strong, with a value of r = 0.818 and P
Conclusion: Based on histopathological findings from the brains of SD rats with TBI, pegagan, cinnamon, and spirulina will protect the brain (neuroprotective) in the acute phase.
Keywords: Centella asiatica, Cinnamon, Neuroprotective, Spirulina, Traumatic brain injury
INTRODUCTION
Brain injury is still a significant health problem throughout the world due to an increasing incidence.[
Traumatic brain injury (TBI) is a condition that occurs when the structure of the head is impacted and causes disruption of brain function.[
According to Basic Health Research in 2018, the prevalence of brain injury in Indonesia is around 11.9%. Injuries to the brain occupy the third most common injury after injuries to the lower limbs and upper limbs, with a respective prevalence of 67.9% and 32.7%. The province of West Nusa Tenggara is the fourth leading contributor to brain injury patients.[
Brain injuries may occur from rotational forces as the brain moves within the skull or can be caused directly by external forces acting on the brain. Before brain damage, persistent cognitive abnormalities were noted in 15–40% of persons.[
In addition, patients may feel fatigue, anxiety, sadness, and neurobehavioral consequences (such as headaches, lightheadedness, and sensitivity to noise). These deficiencies can affect a person’s daily functioning, frequently affecting their quality of life, social interactions, and capacity to return to work.[
Centella asiatica is a wild plant that is efficacious as a traditional medicine for various diseases. Since ancient times, C. asiatica has been used to treat skin problems (e.g., keloids) and nervous disorders and improve blood circulation.[
Cinnamon or Cinnamomum zeylanicum is a spice produced from the dry inner skin, which is very aromatic, sweet, and spicy. People used it in sweet baked foods and hot wine, which was also used as medicine.[
Spirulina is an herbal supplement that contains various vitamins, minerals, and antioxidants that are beneficial for body health.[
Assessing TBI is challenging, but blood glial fibrillary acidic protein (GFAP) can predict intracranial pathologies undetectable on head computed tomography scans.[
This research aimed to examine the neuroprotective properties of C. asiatica, cinnamon, and spirulina in relation to acute TBI. We intend to determine how these natural compounds affect the degree of brain injury and the healing process, focusing on its histological findings. This study attempts to provide insights into new treatment strategies to address the worldwide health burden of TBI.
MATERIALS AND METHODS
We conduct an experimental observational-analytic study by administering medication as an intervention and then analyzing outcomes. This research was done in the Laboratory of the Faculty of Medicine, University of Mataram. Research samples consist of white Wistar rats with the strain Sprague Dawley (SD), with the criteria of the male gender, 10– 12 weeks of age, body weight 300 g, and in good health.
The independent variables in this study were treatment of TBI and C. asiatica, cinnamon, and spirulina. Meanwhile, the dependent variable is the histopathological picture of necrosis markers in the acute phase.
The tools used for hematoxylin and eosin (H&E) staining and Immunohistochemistry (IHC) examination in this study were a slide warmer, antigen retrieval declocking chamber, micropipette, microtome, object glass, cover glass, PAP PEN, tissue, light microscope, and tray.
The materials include paraffin blocks of mice biopsies that had been administered according to their group, xylol I, xylol II, and xylol III, absolute alcohol, 90%, 80%, and 70% alcohol, 0.5% hydrogen peroxide (H2O2), liliemayer’s hematoxylin, and enthelan.
The sample consisted of mice SD, who met the criteria and were randomly divided into two treatment groups: the control group (given a placebo) and the treatment group (given a combination of C. asiatica, cinnamon, and spirulina). The sample was anesthetized with ketamine following craniotomy with a coronal incision. After the dura mater was exposed, the sample was dropped with a weight of 40 g from a height of 20 cm using a Marmarou model. The Marmarou model is a simple brain injury treatment technique.
Mice SD, 3–4 months old, weighing 300 g, were placed in groups (five animals per group) in cages with a room temperature of 25 ± 1°C. Lighting was set on a 12-h light and 12-h dark cycle (the light cycle starts from 6:00 a.m. to 6:00 p.m.). Food and beverages were available in sufficient quantities, acclimatized for 7 days, and eaten and drank ad libitum. Ill or dead experimental animals are excluded from the study. Their body weight was measured, and the mice were assigned to treatment groups randomly.
After all the above was done, the rat’s brain SD will be taken using a micro craniotomy surgical technique and then made into a paraffin block. Paraffin blocks that have been sorted, can be identified, and are not damaged will proceed to the IHC examination stage and H&E staining.
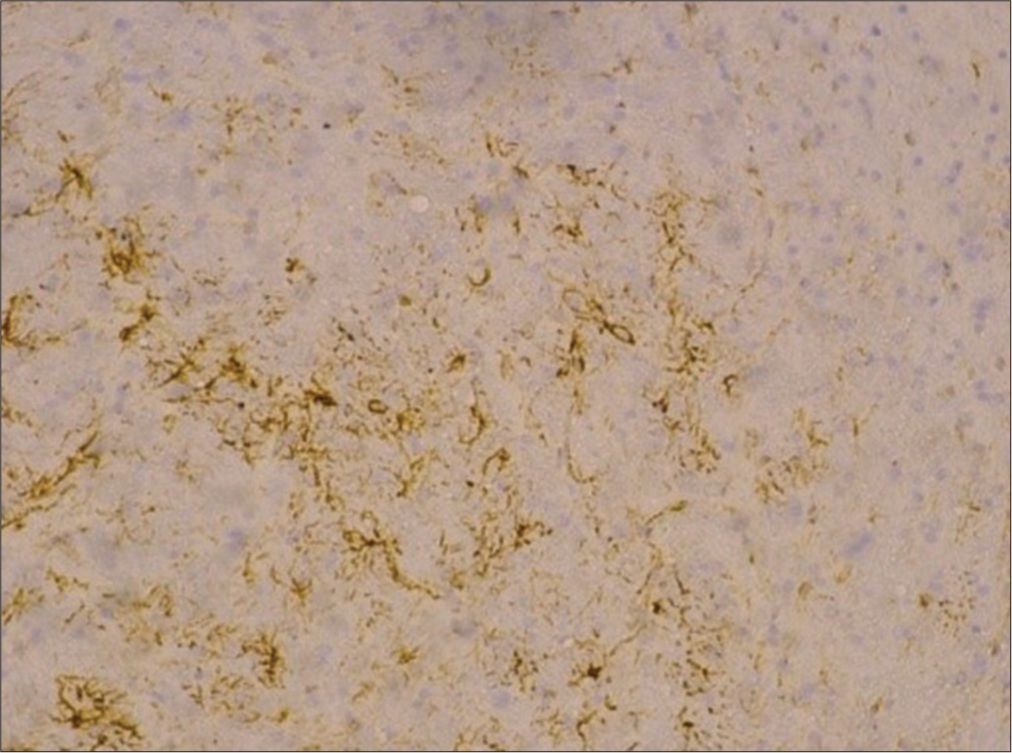
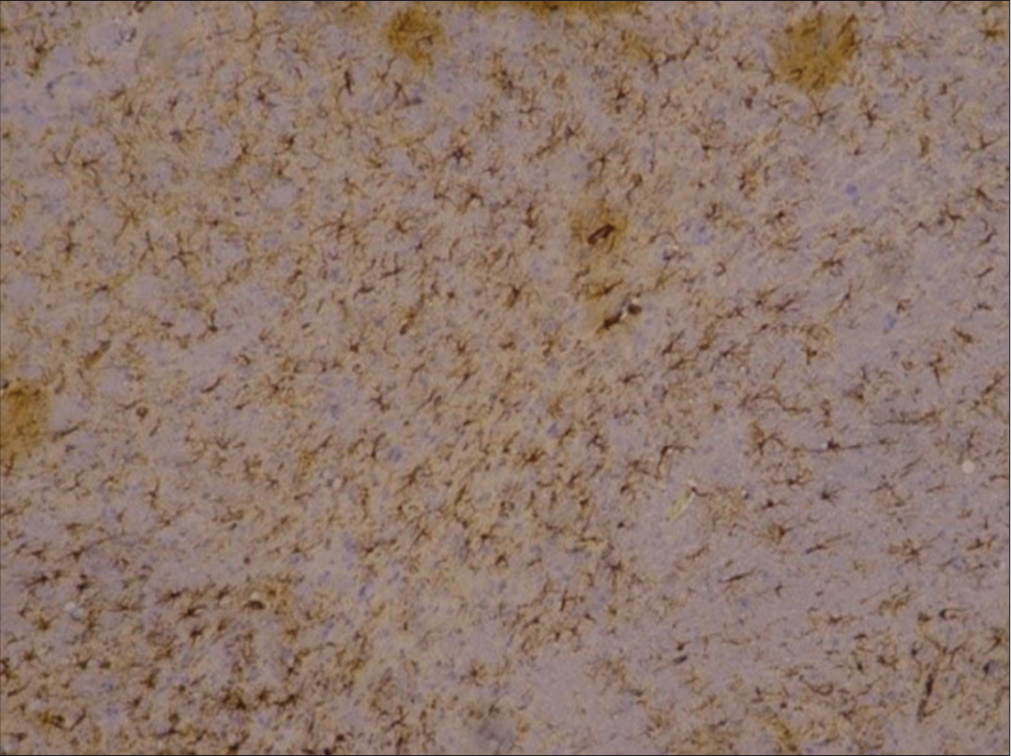
HE samples and paraffin block of rat brain SD were then sent to the anatomy pathology analysis department in Surabaya. The examination began with cutting the H&E paraffin block of rat brain SD 4–6 microns thick, then placing it on a glass object. The immunohistochemistry (IHC) examination carried out is for the acute phase group in the form of GFAP. Then, the preparation was observed with a light microscope with a total magnification of 400 times to interpret the examination. Then, anatomical pathology specialists interpret the preparation. This study used primary data, which was then analyzed using the SPSS computer program.
RESULTS
We did a statistical analysis to obtain an interpretation of the results concerning the conceptual framework and research hypotheses. This study found the mean of Rattus norvegicus strain SD body weight of 290.07 (+10.48) g using the Lavene homogeneity test presented in
Based on
This study uses a brain injury model in Marmarou Modification.[
The association between GFAP during the acute phase and intervention provision was interpreted using a correlation test. In the control group, the intervention dose was considered to be 0; in the intervention group, the dose was 1. The normality test of GFAP for both groups used the Kolmogorov–Smirnov normality test. It was found that both tests were not normally distributed in the intervention group (P < 0.005, 0.003) and the placebo group (P < 0.05, 0.013). Thus, the Spearman test was carried out, and the results of the bivariate test with the Spearman correlation test are presented in
DISCUSSION
Asiatic acid (AA), the most important component of asiaticoside, is present in C. asiatica extracts.[
Figure 4:
The neuroprotective mechanism of Asiatic acid. Nrf2: Nuclear Factor Erythroid 2-related Factor 2, Bcl-2: B-cell lymphoma 2, Bcl-xL: B-cell lymphoma-extra large , Bax: BCL2 Associated X, Apoptosis Regulator, PARP: Poly (ADP-ribose) polymerase, KEAP1: Kelch-like ECH-associated protein 1, NQO1: NAD(P)H dehydrogenase (quinone) 1, HO-1: heme oxygenase , AKT: protein kinase B, PI3K: phosphatidylinositol 3-kinase, mTOR: mechanistic Target of Rapamycin, TNFα:Tumor necrosis factor alpha, IL-1β: interleukin 1-beta , IL-6: interleukin 6, TRAF6: Tumor necrosis factor receptor (TNFR)-associated factor 6 , TAK1Transforming growth factor beta-activated kinase 1, TAB: TAK1-binding proteins, NEMO: NF-κB Essential Modulator , IKKA: IκB kinase, NF-κB: Nuclear Factor kappa B , STAT3: signal transducer and activator of transcription 3, MEK: Mitogen-activated protein kinase/ERK kinase, JAK: Janus Kinase
One of the main causes of disability and mortality, particularly in young adults, is TBI.[
In another study, Gu et al. evaluated the preventive effects of AA on rat craniocerebral damage using the hydraulic shock method to produce an open model and the drop-weight method to create a closed model.[
Cinnamon is a spice that is most commonly used to season food. It has amazing qualities that have helped traditional medicine for millennia by reducing the symptoms of many diseases.[
A study conducted by Qubty et al.[
According to another study by Yulug et al.,[
Spirulina is a popular dietary supplement that is high in antioxidants and proteins. The blue-green algae spirulina is high in proteins, vital fatty acids, vitamins, and minerals. Spirulina is rich in minerals such as manganese, zinc, copper, iron, and gamma-linolenic acid (an important fatty acid), and it includes 60–70% protein by weight. Vitamin B12 is particularly abundant in Spirulina. It also contains a variety of antioxidants, including flavonoids, provitamin A (beta-carotene), Vitamin C, E, selenium, and phycocyanin, as well as SOD, which has been shown to have antioxidant potential in numerous in vivo and in vitro tests.[
According to a study by Thaakur and Sravanthi[
GFAP is a protein that is primarily found in astrocytes (AS) or glial cells, and its presence can induce activation of AS.[
Ho et al., 2013,[
In the study conducted by Chen et al. 2012,[
Rochmah et al., 2019[
Blaylock and Maroon, 2011,[
CONCLUSION
This study showed that the effect of giving C. asiatica, cinnamon, and spirulina on the histopathological findings of the brain of SD rats with TBI could protect the brain (neuroprotective) in the acute phase. C. asiatica, cinnamon, and spirulina can increase GFAP antibody expression in neuron-supporting cells in the acute phase (neuroprotective). There is a correlation between GFAP in histopathological findings and C. asiatica, cinnamon, and spirulina.
Ethical approval
The Institutional Review Board approved the research/study at the Ethical Committee of the Faculty of Medicine, Universitas Mataram, number 048/UN17.F7/ETIK/2023, dated February 16, 2023.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
The authors are very grateful for the guidance to conduct this study.
References
1. Abdelhak A, Foschi M, Abu-Rumeileh S, Yue JK, D’Anna L, Huss A. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022. 18: 158-72
2. Ahmadi A, Naziri M, Fallahpour F, Gholami K, Arabpour J, Pazeshgare F. Therapeutic potential of cinnamon for neurological disorders: A mini-review. Neurol Asia. 2022. 27: 1-17
3. Allen DN, Thaler NS, Cross CL, Mayfield J, editors. Classification of traumatic brain injury severity: A neuropsychological approach. Cluster analysis in neuropsychological research: Recent applications. Germany: Springer; 2013. p.
4. Amoo M, O’Halloran PJ, Henry J, Ben Husien M, Brennan P, Campbell M. Permeability of the blood-brain barrier after traumatic brain injury: Radiological considerations. J Neurotrauma. 2022. 39: 20-34
5. Amorim R, Andrade A, Paiva W, Faleiro R, Monteiro R, Teixeira M. Management of diffuse lesions in traumatic brain injury in Brazil. Austin Neurosurg Open Access. 2014. 1: 1011
6. Blaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis. Surg Neurol Int. 2011. 2: 107
7. Careri M, Furlattini L, Mangia A, Musci M, Anklam E, Theobald A. Supercritical fluid extraction for liquid chromatographic determination of carotenoids in Spirulina Pacifica algae: A chemometric approach. J Chromatogr A. 2001. 912: 61-71
8. CDC (Centers for Disease Control and Preventio, editors. Traumatic Brain Injury. 2019. p.
9. Chen JC, Chan YC, Liu KS, Yang TJ, Hwang JH, Lee IT. Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr Neurosci. 2012. 15: 252-4
10. Cikriklar HI, Uysal O, Ekici MA, Ozbek Z, Cosan DT, Yucel M. Effectiveness of GFAP in determining neuronal damage in rats with induced head trauma. Turk Neurosurg. 2016. 26: 878-89
11. Dawodu ST. Traumatic brain injury: definition, epidemiology, pathophysiology. Med J. 2007. 1:
12. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2019. 130: 1080-97
13. Ding L, Liu T, Ma J. Neuroprotective mechanisms of Asiatic acid. Heliyon. 2023. 9: e15853
14. Finnie JW. Forensic pathology of traumatic brain injury. Vet Pathol. 2016. 53: 962-78
15. Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: Current treatment strategies and future endeavors cell transplantation. Cell Transplant. 2017. 26: 1118-30
16. Gu S, Zhu Q, Wang J, Liu Y, Zhang Y, Rui Y. Protective effects and mechanism of Asiatic acid on traumatic brain injury. J Pharm Pract Serv. 2016. 34: 206-9
17. Han F, Yan N, Huo J, Chen X, Fei Z, Li X. Asiatic acid attenuates traumatic brain injury via upregulating Nrf2 and HO-1 expression. Int J Clin Exp Med. 2018. 11: 360-6
18. Hariri M, Ghiasvand R. Cinnamon and chronic diseases. Adv Exp Med Biol. 2016. 929: 1-24
19. Hariyanto AS, Retnowati E, Turchan A. Serum glial fibrillary acidic protein levels profile in patients with severe traumatic brain injury. Indoneisan J Clin Pathol Med Lab. 2017. 24: 24-8
20. Hickey J, Strayer AL, editors. Clinical practice of neurological and neurosurgical nursing. United States: Wolters Kluwer Health; 2019. p. 816
21. Ho SC, Chang KS, Chang PW. Inhibition of neuroinflammation by cinnamon and its main components. Food Chem. 2013. 138: 2275-82
22. Kamble SM, Patel HM, Goyal SN, Noolvi MN, Mahajan UB, Ojha S. In silico evidence for binding of pentacyclic triterpenoids to Keap1-Nrf2 protein-protein binding site. Comb Chem High Throughput Screen. 2016. 20: 215-34
23. Kastilong M, Subrata II, Tangkudung G, Khosama H. Neutrophyl lymphocyte ratio and the outcome of traumatic brain injury. J Sinaps. 2018. 1: 20-8
24. Khellaf A, Khan DZ, Helmy A. Recent advances in traumatic brain injury. J Neurol. 2019. 266: 2878-89
25. Lalenoh D, Sudjito MH, Suryono B. Anaesthetic management of traumatic brain injury. J Neuroanestesi Indonesia. 2012. 1: 120-32
26. Ma J, Zhang K, Wang Z, Chen G. Progress of research on diffuse axonal injury after traumatic brain injury. Neural Plast. 2016. 2016: 9746313
27. Mioc M, Milan A, Malița D, Mioc A, Prodea A, Racoviceanu R. Recent advances regarding the molecular mechanisms of triterpenic acids: A review (part I). Int J Mol Sci. 2022. 23: 7740
28. Modi KK, Roy A, Brahmachari S, Rangasamy SB, Pahan K. Cinnamon and its metabolite sodium benzoate attenuate the activation of p21rac and protect memory and learning in an animal model of Alzheimer’s disease. PLoS One. 2015. 10: 1-22
29. Mutch CA, Talbott JF, Gean A. Imaging evaluation of acute traumatic brain injury. Neurosurg Clin N Am. 2016. 27: 409-39
30. Nasution RA, Islam AA, Hatta M, Prihantono , Kaelan C, Poniman J. Modification of the Marmarou model in developing countries. Ann Med Surg. 2020. 57: 109-13
31. Ng SY, Lee AY. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019. 13: 528
32. Osier ND, Carlson SW, DeSana A, Dixon CE. Chronic histopathological and behavioral outcomes of experimental traumatic brain injury in adult male animals. J Neurotrauma. 2015. 32: 1861-82
33. Postolache TT, Wadhawan A, Can A, Lowry CA, Woodbury M, Makkar H. Inflammation in traumatic brain injury. J Alzheimers Dis. 2020. 74: 1-28
34. Purwandari KY, Handharyani E, Sajuthi D, Sulistiawati E. Activity of glial fibrillary acidic protein in guinea pig (Cavia porcellus) brain as a model of Alzheimer’s disease with testosterone hormone depletion. Indones J Vet Sci. 2015. 9: 141-6
35. Qubty D, Rubovitch V, Benromano T, Ovadia M, Pick CG. Orally administered cinnamon extract attenuates cognitive and neuronal deficits following traumatic brain injury. J Mol Neurosci. 2021. 71: 178-86
36. Rachmany L, Tweedie D, Rubovitch V, Yu Q, Li Y, Wang J. Cognitive impairments accompanying rodent mild traumatic brain injury involve p53-dependent neuronal cell death and are ameliorated by the tetrahydrobenzothiazole PFT-a. PLoS One. 2013. 8: e79837
37. Rahaman P, Del Bigio MR. Histology of brain trauma and hypoxia-ischemia. Acad Forensic Pathol. 2018. 8: 539-54
38. Reddy CM, Bhat VB, Kiranmai G, Reddy MN, Reddanna P, Madyastha KM. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem Biophys Res Commun. 2000. 277: 599-603
39. Rochmah MA, Harini IM, Septyaningtrias DE, Sari DC, Susilowati R. Centella asiatica prevents increase of hippocampal tumor necrosis factor-α independently of its effect on brain-derived neurotrophic factor in rat model of chronic stress. Biomed Res Int. 2019. 2019: 2649281
40. Rosyidi R, Priyanto B, Januarman Kusdaryono S. Relationship between brain injury severity and inflammatory markers in traumatic brain injury patients at west nusa tenggara provincial hospital. Unram Med J. 2018. 6: 1-4
41. Shavit-Stein E, Gerasimov A, Aharoni S, Gofrit SG, Pikus E, Pick CG. Unexpected role of stress as a possible resilience mechanism upon mild traumatic brain injury (mTBI) in mice. Mol Cell Neurosci. 2021. 111: 103586
42. Thaakur S, Sravanthi R. Neuroprotective effect of Spirulina in cerebral ischemia-reperfusion injury in rats. J Neural Transm. 2010. 117: 1083-91
43. Ulkhaq DLM, Sulistyani , Nursanto D, Setiawan I. Factors Influencing Complications of Traumatic Brain Injury. Publ Ilm Muhammadiyah University Surakarta. 2020. p. 229-33
44. Upasani CD, Balaraman R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phyther Res. 2003. 17: 330-4
45. Vangalapati M, Satya S, Prakash S, Avanigadda S. A review on pharmacological activities and clinical effects of Cinnamon species. Res J Pharm Biol Chem Sci. 2012. 3: 653-63
46. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007. 99: 4-9
47. Williams WH, Chitsabesan P, Fazel S, McMillan T, Hughes N, Parsonage M. Traumatic brain injury: A potential cause of violent crime?. Lancet Psychiatry. 2018. 5: 836-44
48. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013. 14: 128-42
49. Xu L, Nguyen JV, Lehar M, Menon A, Rha E, Arena J. Repetitive mild traumatic brain injury with impact acceleration in the mouse: Multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp Neurol. 2016. 275: 436-49
50. Yulug B, Kilic E, Altunay S, Ersavas C, Orhan C, Dalay A. Cinnamon polyphenol extract exerts neuroprotective activity in traumatic brain injury in male mice. CNS Neurol Disord Drug Targets. 2018. 17: 439-47
51. Zhang S, Wu M, Peng C, Zhao G, Gu R. GFAP expression in injured astrocytes in rats. Exp Ther Med. 2017. 14: 1905-8