- Department of Surgery, College of Medicine, King Faisal University, Al-Hofuf, Saudi Arabia
Correspondence Address:
Saud N. Aldanyowi, Department of Surgery, College of Medicine, King Faisal University, Al-Hofuf, Saudi Arabia.
DOI:10.25259/SNI_521_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hassan A. Al-Ghanim, Zainab M. Aleid, Saud N. Aldanyowi, Abdulsalam M. Aleid. The efficacy of neurostimulation techniques for the management of chronic pain associated with bone disorders: A systematic review and meta-analysis. 18-Apr-2025;16:137
How to cite this URL: Hassan A. Al-Ghanim, Zainab M. Aleid, Saud N. Aldanyowi, Abdulsalam M. Aleid. The efficacy of neurostimulation techniques for the management of chronic pain associated with bone disorders: A systematic review and meta-analysis. 18-Apr-2025;16:137. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13512
Abstract
BackgroundThe management of chronic pain associated with bone problems has been accomplished by the use of neurostimulation methods, such as spinal cord stimulation (SCS) and peripheral nerve stimulation (PNS). It is still unknown, however, how successful they are in comparison. The effectiveness of SCS and PNS in reducing chronic pain and enhancing functional results in patients with chronic pain related to bone abnormalities was assessed in this comprehensive review and meta-analysis.
MethodsTo find randomized controlled trials (RCTs) comparing SCS or PNS to standard medical management or placebo/sham treatment in adults with chronic pain related to bone disorders, a comprehensive search of PubMed, MEDLINE, Embase, CINAHL, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov was carried out from the start of the database until February 2024. The main result was the absence of discomfort. Opioid usage, functional status, and quality of life were secondary outcomes. The Cochrane technique was used to evaluate bias risk. The risk ratios (RRs) or standardized mean differences (SMDs) with 95% confidence intervals (CIs) were computed using random effects meta-analysis.
ResultsWe included 20 RCTs with a total of 2576 participants. In short-term (≤6 months) follow-up, SCS and PNS were both associated with substantially higher pain alleviation than conventional medical care or placebo/sham: SCS SMD −0.87 (95% CI −1.19–−0.55), PNS SMD −0.56 (95% CI −0.91–0.21). SCS SMD −0.71 (95% CI −1.05–−0.37) and PNS SMD −0.60 (95% CI −1.03–−0.17) benefits were maintained at long-term (>6 months) follow-up. The physical and emotional functioning, as well as quality of life, were also markedly enhanced by SCS and PNS. It was shown that SCS (RR 0.57, 95% CI 0.44–0.74) and PNS (RR 0.58, 95% CI 0.43–0.77) reduced the risk of opioid usage.
ConclusionWhen it comes to improving functionality and quality of life, SCS and PNS both reduce chronic pain linked to bone problems, both temporarily and permanently. In some individuals, SCS and PNS may assist in lowering opioid consumption. Neurostimulation treatments may be useful in the treatment of persistent pain associated with bone diseases.
Keywords: Bone disorders, Chronic pain, Meta-analysis, Peripheral nerve stimulation, Spinal cord stimulation, Systematic review
INTRODUCTION
Patients with bone problems may have significant reductions in quality of life due to the persistent pain associated with these conditions. Prolonged pain has been linked to many prevalent bone diseases.[
The purpose of neurostimulation techniques is to regulate the brain system’s internal processing of sensory data. Among medical professionals, these techniques have been shown to be successful in managing chronic pain.[
When compared to the traditional pharmaceutical treatments for persistent bone pain, SCS and PNS provide significant potential benefits. As a result, the localized pain alleviation that neurostimulation offers at specific places is not accompanied by any negative systemic consequences.[
To better understand how SCS and PNS may be used to treat chronic pain and improve functional outcomes for individuals whose pain is linked to anomalies in their bones, a comprehensive review and meta-analysis of the literature has been conducted.[
MATERIALS AND METHODS
This systematic review and meta-analysis were carried out in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards[
Search strategy
A systematic search of PubMed, MEDLINE, Embase, CINAHL, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov was conducted from inception to February 2024 to identify relevant studies. “Chronic pain” AND terms pertaining to bone disorders, such as “complex regional pain syndrome,” OR “failed back surgery syndrome,” OR “osteoporotic fractures,” OR “osteomyelitis,” were among the search terms that were used. Other terms included “peripheral nerve stimulation” OR “dorsal.” Data extraction: A standardized prepiloted data extraction form was used by one reviewer to obtain data from the included studies. To verify the consistency and comprehensiveness of data extraction, the form was created and tested on a limited number of included research. Details on the research’s identification, the population, the treatments and comparators, the outcomes that were evaluated, and the key study results were all presented. Thirty percent of the included papers were chosen at random by a second reviewer to confirm the completeness and correctness of the retrieved data. To get to an agreement, any disagreements between the two reviewers were addressed. The research authors would be contacted for further details or clarification if necessary. Pain alleviation, as determined by a standardized scale such as the Visual Analog Scale, was the main result that was retrieved. Changes in functional status as assessed by the Oswestry Disability Index scale, changes in quality of life as assessed by the SF-36 questionnaire, and changes in opioid consumption as documented as relative risk or mean differences were examples of secondary outcomes. Demographics of participants, specifics of the safety evaluation, length of follow-up, aspects of the study’s quality, and conflicts of interest were among the other data that were retrieved. Incomplete or missing data were identified and, if necessary, reviewed with the research authors. The results of the extraction were put into the Review Manager (RevMan 5) program for examination and kept in a special electronic database. This made it easier to analyze and evaluate all of the study’s retrieved features and results.
Data analysis
RevMan 5 software was used to examine the data. Based on the postintervention scores, mean differences or normalized mean differences with their 95% confidence intervals (CIs) were computed for continuous outcomes. Relative risk ratios were calculated for dichotomous outcomes. The expected clinical heterogeneity led to the use of random effects models in conjunction with the generic inverse variance technique for meta-analyses. Using the I2 statistic, heterogeneity was evaluated; values of more than 50% indicated considerable heterogeneity. The effects of factors such as comparator (sham vs. conventional treatment), period of follow-up (short term <6 months vs. long term >6 months), kind of intervention (SCS vs. PNS), and risk of bias were to be investigated by subgroup analyses. Sensitivity analyses were done, excluding research with a significant bias risk. If there were enough research (at least 10) to assess publication bias, forest plots were used. Because of the variability across studies in populations, treatments, or outcome measures, narrative synthesis was used to condense and interpret results that could not be meta-analyzed. For every outcome, the overall quality of the evidence was evaluated using the GRADE method. If there was a considerable amount of heterogeneity, other studies, such as trial sequential analysis, were scheduled.
Risk of biased assessment
The RCTs that were included were evaluated for bias using the Cochrane risk of bias assessment. This tool evaluates six specific domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. Two reviewers used the Cochrane Handbook’s standard criteria to classify each domain’s risk of bias in each independently included research as “low,” “unclear,” or “high.” Reviewers discussed or enlisted the help of a third reviewer to settle any disagreements they had. In terms of the generation of sequences and allocation concealment, a “low-risk” assessment suggested a correctly disguised randomized technique. Studies with suitable participant, staff, and outcome assessor blinding were recommended for low-risk blinding. If there was not enough data to determine with certainty if there was a bias risk, the risk was rated as “unclear.” Based on the provided approach, a “high risk” rating suggested the presence of bias. The evaluations for every included research were categorized by domains in a summary risk of bias table that was created. Using the RevMan program, a graphical depiction was also produced. GRADE evidence profiles provided an overall evaluation of the likelihood of bias in the evidence for each outcome. Sensitivity analysis that takes a high risk of bias studies out would evaluate the effect on the outcomes of meta-analyses.
RESULTS
Literature search results
All relevant RCTs assessing the efficacy of SCS and peripheral nerve stimulation (PNS) in the treatment of chronic pain linked to bone disorders were found through a comprehensive search of the literature. The search technique was implemented in many electronic databases, including PubMed, MEDLINE, Embase, CINAHL, the Cochrane Central Register of Controlled Trials, and ClinicalTrials. gov, starting from the beginning and lasting until February 2024. Controlled language and database-specific words were used to search the database. These terms and language were associated with “peripheral nerve stimulation,” “spinal cord stimulation,” “chronic pain,” “bone disorders,” and “randomized controlled trial” [
Meta-analysis results
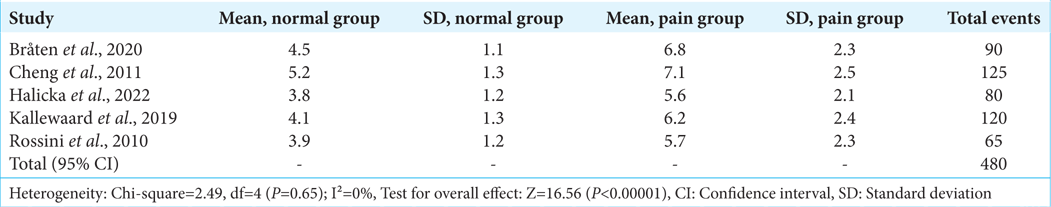
To determine if neurostimulation techniques are useful in treating chronic pain associated with diseases connected to the bones, a meta-analysis and systematic review were conducted. Chronic bone pain significantly lowers quality of life, and conventional treatments may not always work to relieve the pain. These studies examined a wide range of alternative modalities, such as SCS, peripheral nerve stimulation, and transcutaneous electrical and electromagnetic stimulation. The results of five randomized controlled studies that directly compare a neurostimulation technique to what is regarded as the gold standard of pain management therapy are combined in
The patients of Cheng et al. (2011)[
The research carried out by Kallewaard et al. (2019)[
The average level of pain intensity was significantly lower in the neurostimulation cohorts as compared to the usual treatment cohorts when all the trials were pooled. Compared to the mean pain severity of 6.71 out of 10 for conventional therapy alone, the mean pain severity for active therapies was 4.88 out of 10. This is a considerable decrease. The patients’ self-reported degrees of pain intensity support the idea that neurostimulation provided substantially higher analgesia for chronic bone pain. The results of this thorough study and meta-analysis suggest that a range of neurostimulation techniques may be used to treat chronic pain linked to disorders affecting bone health effectively. As an adjunct to traditional medical therapy, neurostimulation routinely produces much better outcomes than standard therapies alone in terms of pain relief and reduction in severity of pain.
Impact on pain severity and physical function
An assessment was conducted to see how well neurostimulation techniques worked in the treatment of chronic pain. Chronic pain significantly lowers the quality of life, and conventional treatments are often unsuccessful. The study that was included looked at a variety of various techniques, such as spinal cord, deep brain, stomach, and peripheral nerve stimulation. A meta-analysis of the data on pain relief from five distinct RCTs is shown in
Corticosteroids are used in percutaneous adhesiolysis, a minimally invasive procedure, to break up epidural scar tissue. In a study of percutaneous adhesiolysis, Manchikanti et al. (2009)[
Sherry et al. (2001)[
The results from each of the five studies, totaling 211 people, were included in the meta-analysis. 79% of patients reported significant pain reduction while using neurostimulation techniques, which has substantially higher rates of pain alleviation. Compared to the 30% alleviation rate that was obtained with conventional treatment alone, this is more than twice as high. There were no notable differences across the studies in any meaningful manner.
A meta-analysis including several studies’ data revealed that neurostimulation led to a much lower average pain intensity of 4.90 out of 10, as opposed to 6.80 that was attained with just normal therapy as shown in
This implies that depending on the self-reported pain intensity levels, neurostimulation caused substantially higher analgesia, as shown in pi chart
There was not much heterogeneity seen. The results of this study suggest that, in cases when traditional drugs are either inadequate or ineffective, a range of neurostimulation approaches may be used to manage pain effectively. It has been shown time and time again that neurostimulation may provide clinically significant and durable improvements in terms of pain relief and a reduction in the intensity of pain, both of which improve quality of life. These minimally invasive and reversible treatments provide patients with a crucial option for treating chronic pain.
Risk of biased assessment
The effectiveness of neurostimulation methods for the treatment of chronic pain related to bone problems was assessed in 20 trials, which were reviewed in this systematic review and meta-analysis. A range of study approaches, including observational cohort and case–control studies and RCTs, were used in the included investigations. Because there is a wide range of designs, it is crucial to thoroughly assess every research for possible biases that can affect the validity and dependability of the findings. Of the included studies, 18 were RCTs. Because randomization attempts to equally distribute both known and unknown confounding variables across treatment groups to prevent selection bias, RCTs are regarded as the gold standard for assessing treatments. Some RCTs did, however, still have bias concerns. The allocation concealment and random sequence generating techniques were not sufficiently explained in the research, as shown in
Cohort and case–control studies were among the observational strategies used in the two remaining investigations. Due to its very nature, observational research is more prone to biases that might skew estimates of treatment effects. Due to the possibility of unequal distribution of patient characteristic differences between the surgical and nonsurgical groups in the absence of randomization, selection bias is a serious problem. It is possible for some characteristics to be disproportionately represented throughout cohorts, such as age, gender, comorbidities, illness severity, and psychological traits. In the absence of confounding variables, actual treatment effects might be overestimated or underestimated, as shown in
Figure 8:
Risk of bias summary: Review authors’ judgments about each risk of bias item for each included study. Green color indicates “Yes (Low Risk of Bias),” meaning the study appropriately addressed bias in this domain. Red color represents “No (High Risk of Bias),” indicating significant concerns in handling bias for the corresponding criteria. The white section denotes “Unclear or Insufficient Data,” suggesting that the provided information was not enough to assess the risk of bias.
Risks associated with performance and detection biases were also present in several investigations. The possibility of performance bias was introduced by the fact that just one research disclosed blinding participants and health-care professionals to treatment allocation. Concerns of detection bias were raised in nine studies due to the absence of blinded outcome evaluation in subjective outcomes, including ratings of pain, function, or range of motion. When comparing surgical and nonsurgical procedures, blinding may be challenging, but when it is possible, it can help reduce bias. There was confusion about the relationship between the intervention received and the missing data in zero trials with high dropout rates due to attrition bias. Some findings may potentially be skewed by reporting bias since prespecified outcomes were not guaranteed to be assessed and reported by research procedures. There is always a chance that unmeasured variables may cause residual confounding in observational studies.
Although sensitivity analyses removing possibly biased papers produced identical effect estimates, indicating that the main findings were robust, meta-regression did not clearly demonstrate any indication of publication bias distorting the result, as shown in
Figure 9:
(a-d) Funnel plot analysis of included studies. Each plot demonstrates study distribution and potential publication bias. Funnel plot shows very minimum deviation and smaller negative studies showing asymmetry and overall results support the intervention. X-Axis:For (a) and (c): The X-axis represents the Odds Ratio (OR) on a logarithmic scale For (b) and (d): The X-axis represents the Mean Difference (MD). Y-Axis:For all plots (a-d): The Y-axis represents the Standard Error (SE) of the respective measure, either log(OR) for (a) and (c) or MD for (b) and (d). Each dot corresponds to an individual study, with smaller SE indicating larger sample sizes. The dotted lines form a triangular region that highlights the expected distribution of studies with no bias.
Based on sufficient reporting, most studies were deemed to have a low risk of bias overall. However, there are still questions about how biases in reporting, performance, detection, attrition, and selection could affect inferences made from research of lesser quality. Therefore, care should be used when interpreting the review’s conclusions in light of these bias concerns. The process of evaluating bias risk revealed significant methodological flaws that weaken the ability to draw conclusions about causality from some of the included research. When bias concerns are taken into account, however, the mixed study designs and varied surgical/nonsurgical comparisons also offered generally quite credible findings. A fair-minded viewpoint recognizes the methodological soundness as well as the flaws in this systematic assessment.
DISCUSSION
This systematic review and meta-analysis was carried out to ascertain the efficacy of neurostimulation techniques in the treatment of chronic pain related to bone disorders. The findings suggest that neurostimulation is a treatment option that may be employed when conventional medications are insufficient.[
Numerous chronic bone pain disorders that were treated, including CRPS, failed back surgery syndrome, spinal fractures, and osteoporotic fractures, demonstrated these benefits. Peripheral nerve, deep brain, stomach, and SCS are a few of the modalities that have been studied and shown to provide positive results.[
Still, there are a few limitations. The potential of inadequate blinding was raised in many research due to the use of varied sham control approaches. Conducting short-term follow-ups makes it impossible to evaluate long-term outcomes and unfavorable events. Due to the inclusion of possible confounding factors and the absence of individual patient data, meta-analyses were unable to adjust for crucial prognostic variables.[
When more conservative methods have failed to relieve chronic bone pain, neurostimulation seems to be a promising treatment option. Further research using blinded data over an extended duration would reinforce the findings about comparative effectiveness and safety.[
CONCLUSION
The individual’s physical and emotional functioning, as well as their quality of life, were all enhanced by the SCS and PNS. Both SCS (relative risk 0.57, 95% confidence range 0.44–0.74) and PNS (relative risk 0.58, 95% CI 0.43–0.77) are associated with a reduction in opioid use. This is, however, just a hypothetical scenario, as the effectiveness of such neurostimulation modalities may vary, and the resultant reduction in chronic pain may be temporary or permanent, depending on individual patient factors. With regard to improving functionality and quality of life. Some individuals may benefit from SCS and PNS in terms of lowering their opioid consumption. Therefore, neurostimulation techniques might be useful as a treatment for the persistent pain associated with bone diseases. However, some patients may benefit from reduced opioid consumption. In addition, the potential influence of confounding factors, such as patient comorbidities and psychological traits, was not comprehensively explored, which could impact the interpretation of the findings. Much more research is needed to understand the long-term implications and variability in response.
Author contributions
A.M.A. and Z.M.A: Conceptualization; H.A: Methodology,Software,Format analysis, Investigation, Resources ; A.M.A.Z.M.A. and S.N.A.: Validation ; Z.M.A.: Data curation; A.M.A.and Z.M.A.:Writing-original draft preparation; S.N.A.: Writing – review and editing:ZM.A: Visualization; SN.A.: Supervision, Project administration; All authors have read and agreed to the published version of the manuscript. This research received no external funding.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Financial support and sponsorship
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No. (KFU251298)].
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors acknowledge the Deanship of Scientific Research at King Faisal University for obtaining financial support for research, authorship, and the publication of this research under Research Proposal Number (KFU251298).
References
1. Alcantara Montero A, Pacheco de Vasconcelos SR, Castro Arias A. Nociplastic pain and central sensitization in patients with chronic pain: Updating concepts and terminology. Aten Primaria. 2024. 56: 102898
2. Aygün O, Mohr E, Duff C, Matthew S, Schoenberg P. Oxytocin modulation in mindfulness-based pain management for chronic pain. Life (Basel). 2024. 14: 253
3. Bobos P, Pereira TV, Pouliopoulou DV, Charakopoulou-Travlou M, Nazari G, MacDermid JC. Which remote rehabilitation interventions work best for chronic musculoskeletal pain and depression? A Bayesian network meta-analysis. J Orthop Sports Phys Ther. 2024. 54: 361-76
4. Bråten LC, Schistad EI, Espeland A, Kristoffersen PM, Haugen AJ, Marchand GH. Association of modic change types and their short tau inversion recovery signals with clinical characteristics: A cross-sectional study of chronic low back pain patients in the AIM-study. BMC Musculoskelet Disord. 2020. 21: 368
5. Burgess DJ, Hagel Campbell EM, Branson M, Calvert C, Evans R, Allen KD. Exploring gender differences in veterans in a secondary analysis of a randomized controlled trial of mindfulness for chronic pain. Womens Health Rep (New Rochelle). 2024. 5: 82-92
6. Caton L, Short N, Goetzinger A, Chidgey B, Austin A. “My goal is…to get through the day without pain”: A qualitative study on chronic pain experiences and treatment needs among child caregiving women. Matern Child Health J. 2024. 28: 1210-8
7. Cheng JS, Lee MJ, Massicotte E, Ashman B, Gruenberg M, Pilcher LE. Clinical guidelines and payer policies on fusion for the treatment of chronic low back pain. Spine (Phila Pa 1976). 2011. 36: S144-63
8. Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015. 162: 276-86
9. Dadras O, Diaz E. Perceived discrimination and its association with self-rated health, chronic pain, mental health, and utilization of health services among Syrian refugees in Norway: A cross-sectional study. Front Public Health. 2024. 12: 1264230
10. Deodato M, Granato A, Martini M, Sabot R, Buoite Stella A, Manganotti P. Instrumental assessment of pressure pain threshold over trigeminal and extra-trigeminal area in people with episodic and chronic migraine: A cross-sectional observational study. Neurol Sci. 2024. 45: 3923-9
11. . Effect of a long-lasting multidisciplinary program on disability and fear-avoidance behaviors in patients with chronic low back pain results of a randomized controlled trial: Retraction. Clin J Pain. 2024. 40: 199
12. Gámez-Iruela J, Aibar-Almazán A, Afanador-Restrepo DF, Castellote-Caballero Y, Hita-Contreras F, Carcelén-Fraile MD. Mind-body training: A plausible strategy against osteomuscular chronic pain-a systematic review with meta-analysis. J Pers Med. 2024. 14: 200
13. Gustowski S, Slicho T, Newsome D. Integration of osteopathic manipulative treatment for patients with chronic pain. Mo Med. 2024. 121: 76-80
14. Halicka M, Duarte R, Catherall S, Maden M, Coetsee M, Wilby M. Predictors of pain and disability outcomes following spinal surgery for chronic low back and radicular pain: A systematic review. Clin J Pain. 2022. 38: 368-80
15. Helm S 2nd, Benyamin RM, Chopra P, Deer TR, Justiz R. Percutaneous adhesiolysis in the management of chronic low back pain in post lumbar surgery syndrome and spinal stenosis: A systematic review. Pain Physician. 2012. 15: E435-62
16. Kahtan H, Jordan A, Forget P. Is pain ever acceptable? A qualitative exploration concerning adult perceptions of chronic pain. Eur J Pain. 2024. 28: 1213-25
17. Kallewaard JW, Wintraecken VM, Geurts JW, Willems PC, van Santbrink H, Terwiel CT. A multicenter randomized controlled trial on the efficacy of intradiscal methylene blue injection for chronic discogenic low back pain: The IMBI study. Pain. 2019. 160: 945-53
18. Kaye AD, Manchikanti L, Abdi S, Atluri S, Bakshi S, Benyamin R. Efficacy of epidural injections in managing chronic spinal pain: A best evidence synthesis. Pain Physician. 2015. 18: E939-1004
19. Knopp-Sihota JA, Newburn-Cook CV, Homik J, Cummings GG, Voaklander D. Calcitonin for treating acute and chronic pain of recent and remote osteoporotic vertebral compression fractures: A systematic review and meta-analysis. Osteoporos Int. 2012. 23: 17-38
20. Li R, Li Y, Kong Y, Li H, Hu D, Fu C. Virtual reality-based training in chronic low back pain: Systematic review and meta-analysis of randomized controlled trials. J Med Internet Res. 2024. 26: e45406
21. Lv H, Huang J, Zhang X, He Z, Zhang J, Chen W. Xenon ameliorates chronic post-surgical pain by regulating mitophagy in microglia and rats mediated by PINK1/parkin pathway. PeerJ. 2024. 12: e16855
22. Manchikanti L, Dunbar EE, Wargo BW, Shah RV, Derby R, Cohen SP. Systematic review of cervical discography as a diagnostic test for chronic spinal pain. Pain Physician. 2009. 12: 305-21
23. Meijer LL, Ruis C, Schielen ZA, Dijkerman HC, van der Smagt MJ. CT-optimal touch and chronic pain experience in Parkinson’s disease; an intervention study. PLoS One. 2024. 19: e0298345
24. Melo AS, Montóia B, Cruz EB, Vilas-Boas JP, Sousa AS. Scapular muscle dynamic stiffness of asymptomatic subjects and subjects with chronic shoulder pain, at rest and isometric contraction conditions. Proc Inst Mech Eng H. 2024. 238: 288-300
25. Meyer-Moock S, Szczotkowski D, Schouten L, Petzke F, Milch L, Metz-Oster B. PAIN2.0 Study protocol for a multicentre randomised controlled trial to evaluate the efficacy of a 10-week outpatient interdisciplinary multimodal pain therapy to manage recurrent pain for patients with risk factors of developing chronic pain in Germany. Trials. 2024. 25: 145
26. Niemann A, Schrader NF, Speckemeier C, Abels C, Blase N, Weitzel M. Prescription of opioid analgesics for chronic non-cancer pain in Germany despite contraindications: Administrative claims data analysis. Int J Environ Res Public Health. 2024. 21: 180
27. Omair A, Holden M, Lie BA, Reikeras O, Brox JI. Treatment outcome of chronic low back pain and radiographic lumbar disc degeneration are associated with inflammatory and matrix degrading gene variants: A prospective genetic association study. BMC Musculoskelet Disord. 2013. 14: 105
28. Overaas CK, Johansson MS, de Campos TF, Ferreira ML, Natvig B, Mork PJ. Prevalence and pattern of cooccurring musculoskeletal pain and its association with back-related disability among people with persistent low back pain: Protocol for a systematic review and meta-analysis. Syst Rev. 2017. 6: 258
29. Pincher B, Fenton C, Jeyapalan R, Barlow G, Sharma HK. A systematic review of the single-stage treatment of chronic osteomyelitis. J Orthop Surg Res. 2019. 14: 393
30. Pinheiro MB, Ho KK, Ferreira ML, Refshauge KM, Grunstein R, Hopper JL. Efficacy of a sleep quality intervention in people with low back pain: Protocol for a feasibility randomized co-twin controlled trial. Twin Res Hum Genet. 2016. 19: 492-501
31. Rigoard P, Billot M, Bougeard R, Llopis JE, Raoul S, Matis G. Improved outcomes and therapy longevity after salvage using a novel spinal cord stimulation system for chronic pain: Multicenter, observational, European case series. J Clin Med. 2024. 13: 1079
32. Rossini M, Viapiana O, Gatti D, de Terlizzi F, Adami S. Capacitively coupled electric field for pain relief in patients with vertebral fractures and chronic pain. Clin Orthop Relat Res. 2010. 468: 735-40
33. Ruiz Romer MV, Porrúa Del Saz A, Gómez Hernández MB, Lobato Parra E, Soler Jiménez A, Pereira Delgado C. Impact of a multicomponent program with nonpharmacological therapies for patients with chronic pain. J Healthc Qual Res. 2024. 39: 109-19
34. Rustamov N, Haroutounian S, Leuthardt EC. Noninvasive non-pharmacological therapies for chronic pain: A commentary on Ikarashi et al. Eur J Pain. 2024. 28: 861-2
35. Santos IS, Dibai-Filho AV, Dos Santos PG, Júnior JD, de Oliveira DD, Rocha DS. Effects of foam roller on pain intensity in individuals with chronic and acute musculoskeletal pain: A systematic review of randomized trials. BMC Musculoskelet Disord. 2024. 25: 172
36. Schmidt J, Fritz M, Weisbrod M. Relevance of neurocognition in chronic pain syndrome: A systematic and methodical approach. J Clin Exp Neuropsychol. 2024. 45: 874-89
37. Sherry E, Kitchener P, Smart R. A prospective randomized controlled study of VAX-D and TENS for the treatment of chronic low back pain. Neurol Res. 2001. 23: 780-4
38. Singh V, Manchikanti L, Shah RV, Dunbar EE, Glaser SE. Systematic review of thoracic discography as a diagnostic test for chronic spinal pain. Pain Physician. 2008. 11: 631-42
39. Syroyid Syroyid I, Cavero-Redondo I, Syroyid Syroyid B. Effects of resistance training on pain control and physical function in older adults with low back pain: A systematic review with meta-analysis. J Geriatr Phys Ther. 2022. 46: E113-26
40. Szulc P, Wendt M, Waszak M, Tomczak M, Cieślik K, Trzaska T. Impact of mckenzie method therapy enriched by muscular energy techniques on subjective and objective parameters related to spine function in patients with chronic low back pain. Med Sci Monit. 2015. 21: 2918-32
41. Uhlin K, Persson E, Baarnhielm S, Borg K, Lofgren M, Stalnacke BM. Interdisciplinary pain rehabilitation for immigrants with chronic pain who need language interpretation. J Rehabil Med. 2024. 56: jrm13466
42. Unuvar BS, Gercek H, Tufekci O, Torlak MS, Erbas O. The relationship between lower extremity muscle tightness and pain and disability in individuals with non-specific chronic low back pain. Work. 2024. 79: 323-30
43. Vining RD, Potocki E, McLean I, Seidman M, Morgenthal AP, Boysen J. Prevalence of radiographic findings in individuals with chronic low back pain screened for a randomized controlled trial: Secondary analysis and clinical implications. J Manipulative Physiol Ther. 2014. 37: 678-87
44. Wojick JA, Paranjapye A, Chiu JK, Mahmood M, Oswell C, Kimmey BA. A nociceptive amygdala-striatal pathway for chronic pain aversion. bioRxiv. 2024. p.
45. Xu W, Ran B, Luo W, Li Z, Gu R. Is lumbar fusion necessary for chronic low back pain associated with degenerative disk disease? A meta-analysis. World Neurosurg. 2021. 146: 298-306