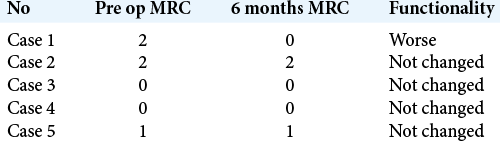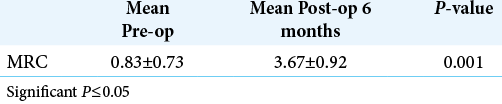- Department of Neurosurgery, Tanta University, Tanta, Egypt,
- Department of Neurosurgery, National Neuroscience Institute, King Fahed Medical City, Riyadh, Saudi Arabia.
- Department of Neurosurgery, King Abdulaziz Specialist Hospital, Taif, Westeran, Saudi Arabia.
- Department of Neurosurgery, Imam Abdulrahman Bin Faisal University, Alkhobar, Saudi Arabia.
- Department of Medicine, College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia.
- Department of Neurosurgery, Royal Commission Hospital, Jubail, Saudi Arabia.
Correspondence Address:
Mohammed Bafaquh, Department of Neurosurgery, National Neuroscience Institute, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_586_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Rizk ElKholy1, Ahmed M. Sallam2, Arwa S. AlShamekh2, Najeeb Alomar2, Fatimah A. Alghabban3, Basmah S. Alzahrani4, Saeed M. Bafaqih5, Fahd A. AlSubaie2, Khalil S. AlQadasi6, Abdulrahman Y. Alturki2, Mohammed Bafaquh2. The efficacy of wrapping the neurorrhaphy site utilizing dura substitute: A case series. 23-Nov-2021;12:568
How to cite this URL: Ahmed Rizk ElKholy1, Ahmed M. Sallam2, Arwa S. AlShamekh2, Najeeb Alomar2, Fatimah A. Alghabban3, Basmah S. Alzahrani4, Saeed M. Bafaqih5, Fahd A. AlSubaie2, Khalil S. AlQadasi6, Abdulrahman Y. Alturki2, Mohammed Bafaquh2. The efficacy of wrapping the neurorrhaphy site utilizing dura substitute: A case series. 23-Nov-2021;12:568. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11247
Abstract
Background: Different procedures have been developed to improve the surgical outcome of peripheral nerve injuries. The purpose of this study was to evaluate the efficacy of wrapping the neurorrhaphy site utilizing dura substitute graft as an alternative conduit in the management of peripheral nerve injury.
Methods: This retrospective clinical case series included 42 patients with a single peripheral nerve injury. The mean age was 26.8 ± 11 years, and the mean duration of symptoms was 3 ± 1.8 months. The visual analogue score (VAS) for pain and the Medical Research Council’s (MRC) grading for motor power were used to evaluate the functional outcome among our patients. All patients were operated on for primary microscopic end-to-end repair, followed by wrapping the neurorrhaphy site with dura substitute graft as a conduit. Patients were followed in the outpatient clinic with regular visits for average of 6 months.
Results: Thirty-seven patients (83%), showed functional improvement in all aspects, the VAS for pain and the MRC for motor power, as well as the functional state. One patient (2.3%) developed a postoperative hematoma collection, which needed immediate evacuation. Superficial wound infection, reported in two patients (4.7%), was treated conservatively. No postoperative neuroma was observed among our patients during the follow-up period.
Conclusion: Wrapping the neurorrhaphy site utilizing dura substitute as conduit appears to be safe and might prove effective in managing peripheral nerve injury.
Keywords: Conduit, Dura substitute, Nerve injury, Neurorrhaphy
INTRODUCTION
Peripheral nerve injury is considered one of the most common clinical problems worldwide, especially in young patients. It affects the quality of life of patients and causes significant a socioeconomic burden.[
Despite improvements in microsurgical techniques for peripheral nerve repair, including epineural neurorrhaphy and interfascicular suturing, only an estimated 50–60% of patients regain useful function after microsurgical repair.[
It is estimated that more than 70% of patients post-nerve injury repair will continue to experience pain for months to years.[
Multiple conduits materials have been developed and utilized in nerve repair, including biological, synthetic tubes, and more recently, tissue-engineered conduits. Some are completely biological, and others are synthetic materials in the form of tubes or conduits. The goal of all types of conduit is to facilitate neurotropic and neurotrophic communication between the proximal and distal stumps of the nerve, with or without a gap in between the stumps, to provide a protective barrier, thus reducing the risk of an impeded healing process due to external factors such as scar tissue formation as well as facilitating nerve tissue regrowth. Although proven effective in varying degrees, the availability of such conduits is generally limited in many medical centers worldwide.[
MATERIALS AND METHODS
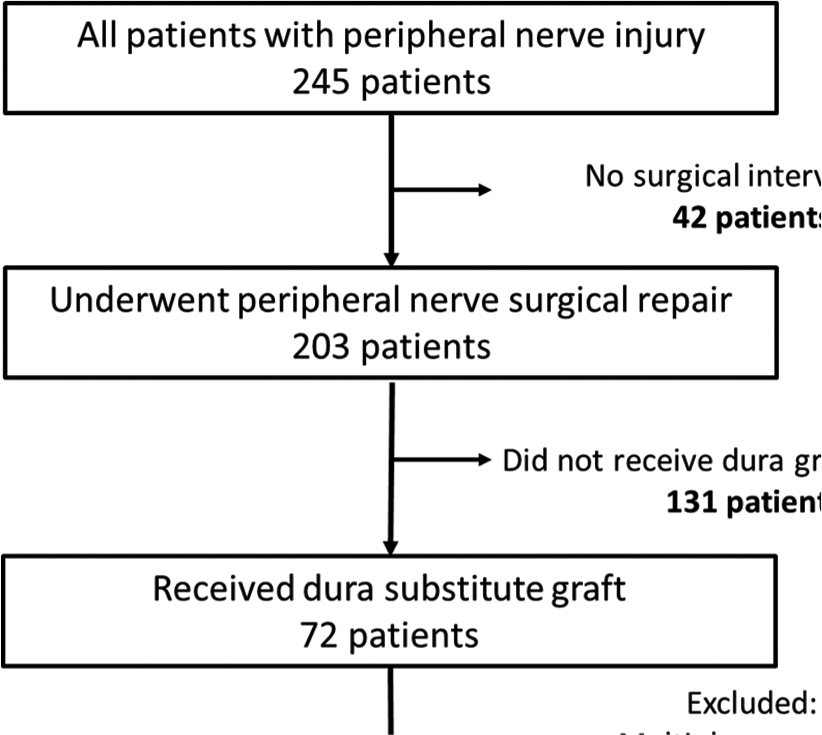
Over a 4 year period (February 2016 to January 2020) hospital record databases were searched for patients with peripheral nerve injuries who had undergone surgical repair at the adult Neurosurgery Department, National Neuroscience Institute, in King Fahad Medical City in Riyadh, Saudi Arabia. Records revealed that 245 patients were seen in our clinic for peripheral nerve injury. Of those 203 patients had undergone microsurgical repair or exploratory procedures related to peripheral nerve injuries including brachial plexus branches, musculocutaneous nerve, median nerve, ulnar nerve, radial nerve, and sciatic nerve branches. Out of the 203 patients, 42 (20%) patients were selected for the study who had undergone microsurgical repair of the injured nerve, utilizing dura substitute graft as a conduit to the injured site [Chart 1]. The remaining patient files were excluded from this study as they did not have a dura substitute as a conduit, had multiple nerve injuries, or nerve graft was used and direct repair was not achieved. Out of the 203 patients, 72 patients had a dura substitute graft; however, 30 patients were excluded as they had their repair after 12 months of the injury (22 patients), or because they had multiple nerve injuries (8 patients). Those patients were not included to avoid factors that might lead to difficulty in understanding or evaluating the efficacy and the safety of an alternative technique to the more expensive and less available conduit material (e.g., the conduit).
The selected 42 patients’ files were reviewed carefully. The involved nerve and level of injury were primarily determined by physical examination, nerve conduction study, and a few patients also had a magnetic resonance imaging (9/42). All selected patients had a single nerve injury, surgical intervention within 6 months from the time of the injury, and direct end-to-end repair was achieved. The remaining 161/203 (79.3%) patients were not included in our analysis as they had the following: (1) multiple nerve injuries; (2) injury more than 6 months’ duration; (3) indirect nerve repair (nerve grafting); and (4) extensive muscle damage or skin loss.
Hospital charts were reviewed and all patients’ data were collected including detailed diagnoses, and treatment outcomes in terms of pain scoring, power, and functionality of the involved limb were collected for analysis [
Approval from the research ethics committee was obtained. However, being a retrospective study, patient consent for participation and publication was not applicable.
Preoperative evaluation
All patients were evaluated and subjected to a complete clinical history and general and neurological examination. Pain was evaluated by the Visual Analogue Scale (VAS).[
The electrophysiological study included electromyography and nerve conduction velocity for all patients, to confirm the diagnosis and identify the site and degree of injury. Nine patients (21.4%) had an MRI scan.
Operative technique
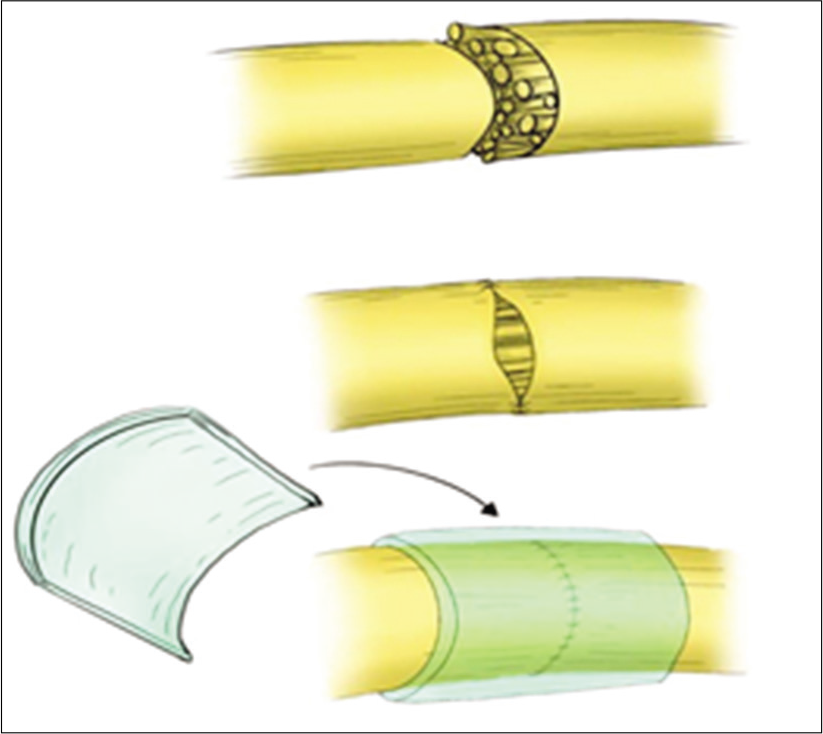
Under general anesthesia, the suspected nerve injury was explored in the usual fashion. The proximal and distal stumps were identified, in some cases after the removal of the associated neuroma, and debrided utilizing a microsurgical technique. Mobilization of both nerve segments was performed as necessary to reduce tension at the repair site [
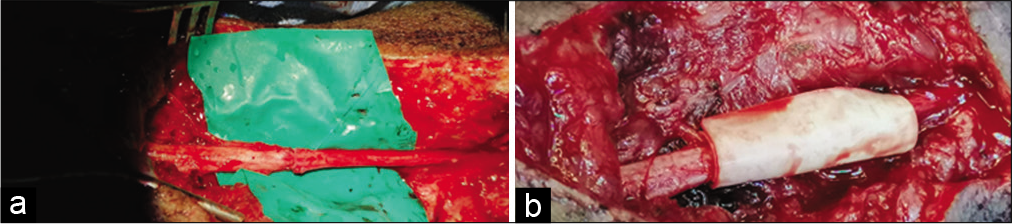
Microscopic repair by epineural sutures occurred for all injured sites, using 6–0 proline suture in most cases, followed by wrapping the repair site with one of the available dura substitute grafts as a conduit; for example., DuraGuard, Tampa, FL, USA; DuraFoam, Menlo Park, CA, USA; Integra/ DuraGen, Plainsboro, NJ, USA; DuraMatrix, Oakland, CA, USA; and Hemopatch/Baxter International, Deerfield, IL, USA [
Postoperative evaluation
As per our standard postoperative follow-up, all patients were seen in the clinic 2 weeks postoperatively to assess the wound, remove the sutures and the cast when applicable, and get a baseline post-op physical exam. The patients are then sent for an extensive rehabilitation program usually spanning 6 weeks, followed by an in-home rehabilitation program. Clinical follow-up was done regularly every 3 months afterward, for at least 12 months, to assess VAS for pain and MRC for motor power. In addition, other tests, including Tinel’s sign at the repair site, were utilized to evaluate the presence of local neuroma formation.
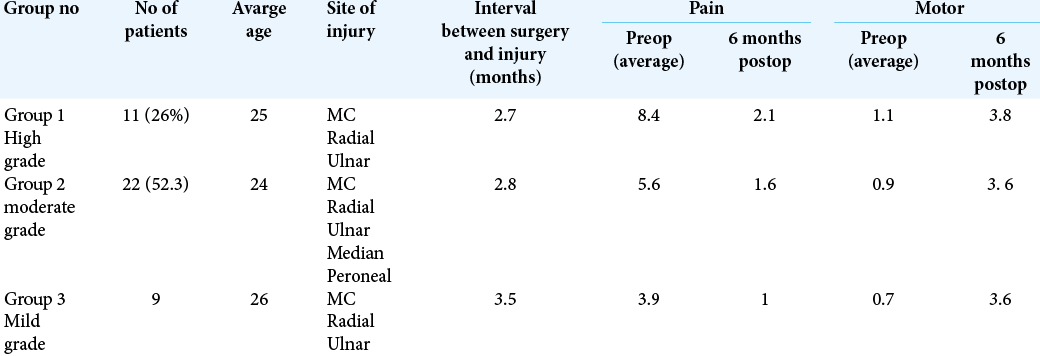
Patient data were collected retrospectively after the 6-month follow-up. Patients were divided into three groups based on the severity of pain and the weakness they reported before the surgical intervention [
RESULTS
This study included 42 patients with a single peripheral nerve injury who underwent a surgical repair. The patient data were collected retrospectively and the average follow-up was found to be at least 9 months postoperatively (minimum follow-up is 6 months and maximum 12 months). Twenty-two patients (52.4%) were males and 20 patients (47.6 %) were females, with a male to female ratio of 1.1: 0.9, and a mean age of 26.8 ± 11 years [
The series included distal (lateral cord) brachial plexus injury in 12 patients (28.6%), musculocutaneous nerve injury in five patients (11.9%), median nerve injury in three patients (7.1%), radial nerve injury in five patients (11.9%), ulnar nerve injury in five patients (11.9%), sciatic nerve injury in seven patients (16.7%), and common peroneal nerve injury in five patients (11.9%).
The onset of injuries to the time of surgical intervention ranged from 1 to 6 months, with a mean duration of 3.0 ± 1.8 months [
The data showed that all patients were operated on for primary microscopic end-to-end repair, followed by site wrapping (neurorrhaphy) with dura substitute conduit: DuraGuard in eight patients (19%), DuraGen in 12 patients (28.6%), DuraFoam in seven patients (16.7%), DuraMatrix in six patients (14.3%), and Hemopatch in nine (21.4%). The dura substitute grafts are made from the same basic material and they are all collagen-rich grafts.[
In the 3- and 6-month follow-ups, the patient record showed that all patient VAS for pain had improved. The mean postoperative VAS at 3 months was 3.29 ± 1.48 and at 6 months was 1.55 ± 0.83. Statistical analysis revealed a significant improvement in VAS among patients at 6 months postoperatively compared to the situation preoperatively [
The MRC for motor power showed overall improvement in most patients 37 (83%); however, one patient (2.3%) had worsening of his strength, from Grade 2 to Grade 0. Four patients (9.5%) did not show any improvement in strength [
Post-operative complications
Patient record showed that postoperative neuroma was not reported among our patients during the follow-up period.
One patient developed postoperative swelling at the surgical wound due to hematoma collection, which necessitated immediate evacuation. Postoperative course was excellent.
Superficial wound infection was reported in 2 patients (4.7%) after surgery, which was treated with oral antibiotics for 10 days.
There was no report of any allergic reaction from the dura substitutes used in our study.
DISCUSSION
The management of peripheral nerve injuries can be difficult and requires a multidisciplinary approach. The objectives of the management must be clear to the treating team and the patient, with the goal of restoring functionality in terms of power, sensation and pain relief, which is a common symptom that can be devastating. In our series, most of the patients (80%) had a neuropathic pain similar to that reported in the literature (60–90%).[
It was noticed that isolating that covering the nerve with the conduits from the external adjacent environment at the repair site can help with the healing process and slow down the diffusion of trophic and modified growth factors. It showed a more promising outcome by potentially reducing the scar formation around the injured site. It also provides a proper environment for regeneration of the injured nerve and acts as a scaffold for cell adhesions and axonal regeneration.[
In Zhang et al.’s 2013 study,[
In 2018, Zhu et al.[
In this work, we used a collagen dura substitute as a conduit to cover the neurorrhaphy site in 42 patients, which resulted in acceptable functional outcomes, including pain and motor power, which denotes proper nerve regeneration [
Neuroma was not seen among our patients during follow-up physical examination. As such, we agree with the results of Thirumalai et al., as they reported that the use of a biologically inert barrier to protect the repair site from surrounding tissues is essential in preventing nerve tether, axonal escape, and neuroma formation.[
Limitations
This is a retrospective study with a limited number of patients and experience of dura substitute graft in peripheral nerve injury. Although this study found that covering the injured nerve postrepair significantly reduced the severity of the pain, we acknowledge that the improvement in strength and overall functionality is not necessarily related to the covering of the nerve but to the selectivity of patients with fresh single nerve injuries.
CONCLUSION
Wrapping the neurorrhaphy site with dura substitute as an alternative conduit can be effective and safe in the management of peripheral nerve injury. We noticed better pain relief with its use, and a similar complication profile to the standard procedure.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
None.
References
1. Battiston B, Geuna S, Ferrero M, Tos P. Nerve repair by means of tubulization: Literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005. 25: 258-67
2. Glück T. About neuroplasty by way of transplantation. Arch Klin Chir. 1880. 25: 606-16
3. Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomat. 2014. 35: 6143-56
4. Huckhagel T, Nüchtern J, Regelsberger J, Lefering R, TraumaRegister DGU. Nerve injury in severe trauma with upper extremity involvement: Evaluation of 49,382 patients from the TraumaRegister DGU® between 2002 and 2015. Scand J Trauma Resusc Emerg Med. 2018. 26: 76
5. John J. Grading of muscle power: Comparison of MRC and analogue scales by physiotherapists. Medical research council. Int J Rehabil Res. 1984. 7: 173-81
6. Kleinert HE, Tsai TM. Microvascular repair in replantation. Clin Orthop Relat Res. 1978. 133: 205-11
7. Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 1999. 8: 243-52
8. Lovaglio AC, Socolovsky M, di Masi G, Bonilla G. Treatment of neuropathic pain after peripheral nerve and brachial plexus traumatic injury. Neurol India. 2019. 67: S32-7
9. MacEwan MR, Kovacs T, Osbun J, Ray WZ. Comparative analysis of a fully-synthetic nanofabricated dura substitute and bovine collagen dura substitute in a large animal model of dural repair. Interdiscip Neurosurg. 2018. 13: 145-50
10. Mailänder P, Berger A, Schaller E, Ruhe K. Results of primary nerve repair in the upper extremity. Microsurgery. 1989. 10: 147-50
11. Martini R, Fischer S, Lopez-Vales R, David S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008. 56: 1566-77
12. Menorca RM, Fussell TS, Elfar JC. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013. 29: 317-30
13. Miclescu A, Straatmann A, Gkatziani P, Butler S, Karlsten R, Gordh T. Chronic neuropathic pain after traumatic peripheral nerve injuries in the upper extremity: Prevalence, demographic and surgical determinants, impact on health and on pain medication. Scand J Pain. 2019. 20: 95-108
14. Millesi H. Interfascicular nerve grafting. Orthop Clin North Am. 1970. 2: 419-35
15. Nickel FT, Seifert F, Lanz S, Meihofner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012. 22: 81-91
16. Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998. 45: 116-22
17. Novak CB, Katz J. Neuropathic pain in patients with upper extremity nerve injury. Physiother Can. 2010. 62: 190-201
18. Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A. Nerve conduits for peripheral nerve surgery. Plast Reconstr Surg. 2014. 133: 1420-30
19. Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013. 9: 668-76
20. Thirumalai A, Jose RM, Power D. The efficacy of vein ensheathing in protecting peripheral nerve repair sites. J Musculoskelet Surg Res. 2019. 3: 123-7
21. Wewers ME, Lowe NK. A critical review of visual analogue scales in measurement of clinical phenomena. Res Nurs Health. 1990. 13: 227-36
22. Wu H, Zhang J, Luo Y, Wan Y, Sun S. Mechanical properties and permeability of porous chitosan-poly (p-dioxanone)/silk fibroin conduits used for peripheral nerve repair. J Mech Behav Biomed Mater. 2015. 50: 192-205
23. Yu Y, Zhang P, Yin X, Han N, Kou Y, Jiang B. Specificity of motor axon regeneration: A comparison of recovery following biodegradable conduit small gap tubulization and epineural neurorrhaphy. Am J Transl Res. 2015. 7: 53-65
24. Zhang P, Han N, Wang T, Xue F, Kou Y, Wang Y. Biodegradable conduit small gap tubulization for peripheral nerve mutilation: A substitute for traditional epineurial neurorrhaphy. Int J Med Sci. 2013. 10: 171-5
25. Zhu X, Wei H, Zhu H. Nerve wrap after end-to-end and tension-free neurorrhaphy attenuates neuropathic pain: A prospective study based on cohorts of digit replantation. Sci Rep. 2018. 8: 620