- Department of Neurological Surgery, University of Miami, Miller School of Medicine, Lois Pope Life Center, Miami, United States
- Department of Neurological Surgery,University of Southern California, Los Angeles, CA, United States
- Department of Otolaryngology, University of Miami, Miller School of Medicine, Lois Pope Life Center, Miami, United States
Correspondence Address:
Muhammet Enes Gurses, MD Department of Neurological Surgery, University of Miami Miller School of Medicine, Lois Pope Life Center, 1095 NW 14th Terrace (D4-6), Miami, FL 33136, USA.
DOI:10.25259/SNI_750_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Muhammet Enes Gurses1, Elif Gökalp2, Neslihan Nisa Gecici1, Khushi Hemendra Shah1, Stephanie Rose Baboun1, Tiffany Alyssa Eatz1, Mynor Mendez Valdez3, Meredith Claire Costello1, Caleigh Samantha Roach1, Martin A. Merenzon1, Victor M. Lu1, Ashish H. Shah1, Michael E. Ivan1, Zoukaa Sargi3, Ricardo J. Komotar1. The learning curve and outcomes of 1038 endoscopic endonasal transsphenoidal pituitary tumor surgeries – A single surgical team experience. 08-Nov-2024;15:407
How to cite this URL: Muhammet Enes Gurses1, Elif Gökalp2, Neslihan Nisa Gecici1, Khushi Hemendra Shah1, Stephanie Rose Baboun1, Tiffany Alyssa Eatz1, Mynor Mendez Valdez3, Meredith Claire Costello1, Caleigh Samantha Roach1, Martin A. Merenzon1, Victor M. Lu1, Ashish H. Shah1, Michael E. Ivan1, Zoukaa Sargi3, Ricardo J. Komotar1. The learning curve and outcomes of 1038 endoscopic endonasal transsphenoidal pituitary tumor surgeries – A single surgical team experience. 08-Nov-2024;15:407. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13208
Abstract
Background: Pituitary adenomas rank third among adult intracranial tumors, with an incidence of 3.9– 7.4 cases/per 100,000 annually. Transsphenoidal surgery has evolved to include endoscopic endonasal surgery (EEA) in many centers due to technological and surgical advancements over the past two decades. We aim to analyze a 12-year cohort of pituitary adenomas operated through EEA, highlighting the evolution of surgical techniques and outcomes.
Methods: A retrospective review of patients undergoing EEA was conducted. A team of an otolaryngologist and neurosurgeon performed surgeries. The cohort was divided into three groups: Phase 1 (P1, 2012–2015), Phase 2 (P2, 2016–2019), and Phase 3 (P3, 2020–2023). Patient demographics, clinical data, and outcomes were collected from electronic medical records and compared over time.
Results: The mean age was 54.2 years, with 53.5% being female. The gross total resection rate was 75.6%, increasing from 62.3% in P1 to 76.3% in P3 (P = 0.003). The mean operative duration was 274.61 min, with no significant correlation to case number. Complication rates, excluding cerebrospinal fluid (CSF) leaks, were similar between the groups, with no statistically significant differences observed for complications such as visual deficit, cranial nerve palsy, and epistaxis. However, meningitis decreased significantly from 3.8% to 0.3% (P P = 0.003). The need for revision surgery was lower in P3 (8.5% vs. 5.4% vs. 2.1, P P
Conclusion: Our experience with EEA for pituitary adenomas shows significant improvements in surgical outcomes, reduced complications, and better postoperative management, underscoring the importance of experience, technical refinement, and a multidisciplinary approach.
Keywords: Adenoma, Endonasal, Endoscopic, Learning curve, Pituitary
INTRODUCTION
Pituitary adenomas are the third most common intracranial tumor in adults, with an incidence rate between 3.9 and 7.4 cases/100.000/year.[
The objective of this study is to analyze our historical 12-year cohort of pituitary adenomas operated through EEA to describe how surgical technical variables and clinical outcomes have evolved and experienced.
MATERIALS AND METHODS
We retrospectively reviewed all pituitary tumor patients who were operated on with EEA between 2012 and 2023 at our institution. A 2-surgeon approach was employed for all operations, involving the same neurosurgeon and otolaryngologist. The study was conducted with the Institutional Review Board approval (20160437), and patient consent was not required due to the retrospective nature of the study and the removal of all identifying patient information.
Electronic medical records were reviewed for patient demographics, preoperative clinical deficits, diagnosis, preoperative and postoperative magnetic resonance imaging (MRI) scans, and lesion characteristics, including location, volume, extent of resection, postoperative complications, and postoperative deficits.
Radiologic assessment
All patients underwent high-resolution MRI scans with and without contrast, specifically focusing on thin sections through the sella. Adenoma volume was calculated using the formula (length × width × height)/2, assessed based on the greatest dimensions in axial, coronal, and sagittal planes from MRI scans. Adenomas were categorized based on the maximum tumor diameter into two groups: Microadenomas (≤10 mm) and macroadenomas (>10 mm). The Knosp classification was utilized to assess the relationship of adenoma with the cavernous sinus.[
Tumors were analyzed using contrast-enhanced MR images on postoperative day 1, 3 months after surgery, and then at a yearly interval. The absence of any detectable tumor on postoperative imaging was defined as “Gross-total resection (GTR).” Progression was characterized by a more than 25% increase in the volume of residual tumor post-STR compared to its dimensions observed on early postoperative imaging. Regression was defined as a minimum 25% reduction in volume during subsequent follow-ups after STR.
Endocrinologic evaluation
Patients received comprehensive evaluation at a specialized multidisciplinary pituitary center, comprising neurosurgeons and endocrinologists. Each patient underwent an initial assessment involving baseline pituitary hormone profiling encompassing serum cortisol, free thyroxine, thyroid-stimulating hormone, adrenocorticotropic hormone (ACTH), growth hormone (GH), insulin-like growth factor, prolactin (PRL), luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone (in men), and estradiol (in women). Endocrine outcomes were assessed based on specific criteria for defining complete biochemical remission in different tumor types. This included the normalization of serum PRL levels (<20 μg/L) for prolactinomas, the normalization of serum GH (<1 μg/L) or nadir GH after an oral glucose tolerance test (≤0.4 μg/L) for GH-producing tumors, and morning serum cortisol levels (<3 μg/dL) 3–5 days postsurgery for ACTH-producing tumors.
Statistical analysis
A comparison of categorical variables was conducted using Chi-square and Fisher exact tests where appropriate. Comparison of continuous variables was conducted using the independent samples t-test and one-way analysis of variance test, and pairwise comparisons were conducted using post hoc testing. Spearman correlation and regression analyses were conducted to assess the relationships between the operation duration and preoperative volume, as well as the number of cases and cerebrospinal fluid (CSF) leaks. A P < 0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences 23.0 software (IBM, New York) and GraphPad Prism software (Version 10.1.2, GraphPad Software Inc., San Diego, California).
RESULTS
Patient characteristics
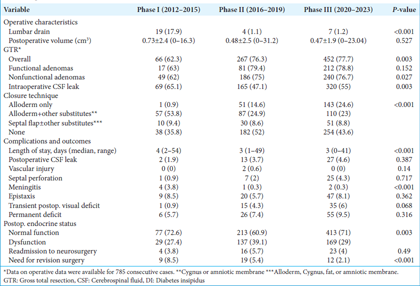
We included 1038 consecutive patients treated over 12 years. The cohort was divided into three groups: Phase 1 (P1, 2012–2015), Phase 2 (P2, 2016–2019), and Phase 3 (P3, 2020–2023). There were 106 patients in P1, 350 patients in P2, and 582 patients in P3, indicating an increase in case volume as the surgeon gained experience. The demographic characteristics of the cohort are summarized in
Among 1038 pituitary adenomas, 387 (37.3%) were functional, and functional adenomas were more frequent in Phase 3 (25.5% vs. 28.6% vs. 44.7%, P < 0.001). Of the functional adenomas, 31% (n = 120) were ACTH-secreting, 29.5% (n = 114) were prolactinoma, 18.9% (n = 73) were GH-secreting, and 12.4% (n = 48) were FSH/LH-secreting adenomas. The majority of the adenomas (87%, n = 903) were located in the sellar region, while 56 (5.4%) were found in the suprasellar region, and 72 (6.9%) were located in both sellar and suprasellar region. According to the Knosp classification, there were 217 (20.9%) Grade 0 adenomas, 247 (23.8%) Grade 1, 172 (16.6%) Grade 2, 256 (24.7%) Grade 3A, 61 (5.9%) Grade 3B, and 85 (8.2%) Grade 4 adenomas. Grade 3–4 adenomas were more frequent in the first group (70.8% vs. 31.4% vs. 37.3%, P < 0.001). Optic chiasm compression was present in 64.9% (n = 674) of the cases and more prevalent in the first group (86.2% vs. 63.5%, P < 0.001). The mean preoperative volume was 6.04 ± 10.49 cm3. Smaller lesions (<3 cm3) were more prevalent in the second and third phases (38.8% vs. 54.8% vs. 54.4%, P = 0.032) [
Operative characteristics and outcomes
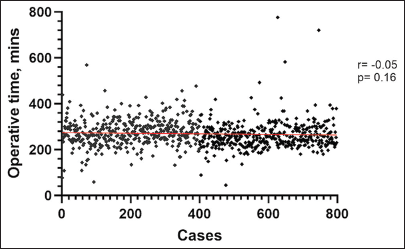
The data on operative duration were available for 785 consecutive cases and were not available for the first 240 cases. Operative duration is defined as the time elapsed from the initial incision to completion of reconstruction. The mean operative duration was 274.61 ± 77.83 min, and there was no correlation between operative duration and the number of cases (r = −0.05, P = 0.16) [
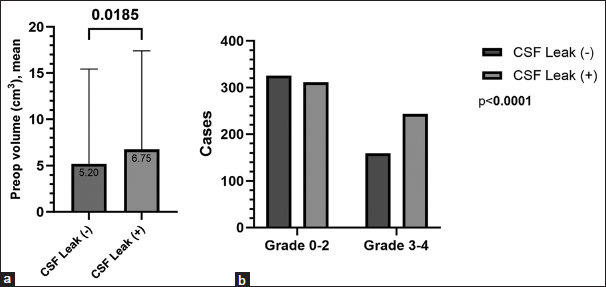
The mean length of hospitalization was 4.06 ± 3.47 days and significantly shorter in the second group (5.3 days vs. 3.8 days vs. 3.9 days, P < 0.001). The intraoperative CSF leak rate was significantly higher in the first group compared to the rest of the cases (65.1% vs. 47.1% vs. 55%, P = 0.003). The mean preoperative volume was significantly greater in the patients with intraoperative CSF leak (6.75 cm3 vs. 5.2 cm3, P < 0.001) [
Epistaxis (7.3%), transient postoperative visual deficits (4.9%), and CSF leak (4.04%) were the three most common postoperative complications. There were no statistically significant differences in rates of complications between the groups, except for the rate of meningitis, which significantly reduced to 0.3% in the third phase from 3.8% in the first phase (P < 0.001). Complications are summarized in
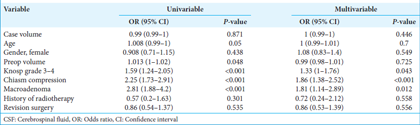
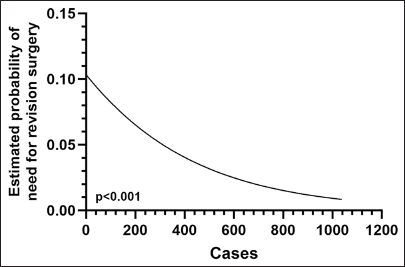
The rate of postoperative permanent deficits, such as visual disturbances or cranial nerve deficits, was 5.7%, 7.4%, and 9.5 in the first, second, and third groups, respectively (P = 0.316). Among the patients, 67.7% had normal endocrine function postoperatively, with 72.6% in the first group, 60.9% in the second group, and 71% in the third group (P = 0.003). Among the patients who had functional adenomas, the percentage of patients with normal endocrine function in the postoperative period was 63% in the first group, 58.8% in the second group, and 63.2% in the third group (P = 0.737). The need for revision surgery was significantly lower in Phase 3 (8.5% vs. 5.4% vs. 2.1%, P < 0.001). There was a significant association between the risk of the need for revision surgery and case volume (r = −0.131, OR: 0.997 [95% CI: 0.996–0.999], P < 0.001) [
DISCUSSION
The steep initial learning curve in endoscopic transsphenoidal surgery cases has been reported in the literature.[
The traditional learning curve exhibits an S-shaped progression comprising three distinct stages. The initial phase encompasses a gradual acquisition of new skills, followed by a phase characterized by rapid proficiency enhancement. The final stage marks a plateau, signifying the attainment of mastery.[
The significant improvement in the rate of GTR, particularly for nonfunctional adenomas, from 62% in the first group to 76.7% in the third group (P = 0.027), underscores the significant advancement in surgical skills and experience. Nonfunctional adenomas tend to be larger and more invasive due to their slower growth and later presentation. The primary surgical goal for nonfunctional adenomas is to resect as much of the tumor as possible while minimizing harm to surrounding structures, especially given their proximity to critical areas such as the optic chiasm and cavernous sinus. The significant improvement in GTR rates for nonfunctional adenomas reflects enhanced surgical techniques, better preoperative planning, and refined intraoperative strategies over time.
The rate of intraoperative CSF leaks was notably higher in Phase 1 (65.1% vs 47.1% vs 55%, P = 0.003), which aligns with the learning curve associated with mastering endoscopic techniques and reflects the refinement of our skills in preserving the integrity of the diaphragma sella while ensuring GTR. Several studies noted a similar reduction in CSF leaks with increased case volume.[
Intraoperative CSF leak was more common in Knosp grade 3–4 adenomas (61.7%) because these adenomas often invade the cavernous sinus and have a higher likelihood of involving the diaphragma sellae. Although parasellar invasion typically does not involve the diaphragma sellae directly, the adenoma’s lateral extension and displacement of surrounding structures increase the risk of dural tears, especially during resection. In such cases, inadvertent rupture or thinning of the diaphragm or adjacent sellar dura can lead to a CSF leak. In addition, in high-grade adenomas, the tumor’s proximity to the suprasellar cisterns increases this risk. To address adenoma invasion into the cavernous sinus, we utilized a transsellar approach, following the tumor from the sella into the cavernous sinus. This technique allows gradual tumor debulking and minimizes trauma to surrounding neurovascular structures. We did not rely solely on diaphragmatic rupture; instead, we improved exposure by lateralizing the pituitary gland and selectively resecting the medial wall of the cavernous sinus where needed.[
While overall complication rates remained comparable between the two groups, the incidence of meningitis significantly decreased from 3.8% in the first group to 0.3% in the remaining cohort (P < 0.001). This reduction likely reflects improvements in surgical technique, aseptic protocols, and perioperative management. The need for revision surgeries was also significantly lower in the latter cohort (8.5% vs. 5.4% vs. 2.1%, P < 0.001), suggesting enhanced initial surgical efficacy and better long-term outcomes. Moreover, a significant reduction in the length of hospitalization was observed, decreasing from an average of 5.3 days in the first group to 3.9 days in the third group (P < 0.001). This reduction can be attributed to more efficient surgical procedures, enhanced postoperative care, and the benefits of a minimally invasive approach.
The postoperative endocrine function was another measure of success. Although a higher percentage of patients in the first phase retained normal endocrine function postoperatively (72.6% vs 60.9% vs. 71%, P = 0.003), this discrepancy might be influenced by the selection of less complex cases during the initial phase of the learning curve. Notably, functional adenomas were more frequent in the third group (25.5% vs 28.6% vs 44.7%, P < 0.001), and the surgical resection of these hormone-secreting adenomas is often a more complex procedure associated with higher complication rates and a strict necessity for GTR.[
A critical aspect contributing to the success of these surgeries and the mitigation of complications, especially intraoperative and postoperative CSF leaks, is the consistent involvement of the same surgical team, comprised of the same neurosurgeon and otolaryngologist, throughout all 1038 cases. This collaboration facilitates a seamless working relationship, allowing for refined coordination and shared expertise, ultimately contributing to the positive outcomes observed in our study. Likewise, Lofrese et al. emphasized the significance of collaboration with otolaryngologists in endoscopic pituitary surgery.[
Limitations
A significant limitation of our study is its retrospective design, inherently carrying the risk of selection bias and the inability to control or standardize data collection methods. The observed decrease in the operative duration between the two halves may have limited clinical relevance. In addition, the surgeries were conducted at a single center, potentially limiting the generalizability of the findings to other institutions. Moreover, the process of assimilating a new surgical technique, akin to any cognitive endeavor, is shaped by a multitude of influences. The expertise of the surgeon, accumulated experience, team dynamics, and the impact of emerging tools and technological advancements collectively contribute to the outcome. Whether this trajectory will persist or reach a stabilization phase remains uncertain, awaiting further observation over time. Notably, existing literature implies that the surgical learning curve might extend beyond a decade of ongoing refinement.[
CONCLUSION
In summary, our extensive experience with endoscopic endonasal transsphenoidal surgery for pituitary adenomas demonstrates a clear learning curve, with significant improvements in surgical outcomes, reduction in complications, and enhanced postoperative management over time. This study underscores the importance of experience, technical refinement, and a multidisciplinary approach in optimizing the outcomes for patients undergoing this complex surgical procedure.
Ethical approval
The research/study was approved by the Institutional Review Board at the University of Miami, number 20160437, dated 2022.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ben-Shlomo N, Mudry A, Naples J, Walsh J, Smith TR, Laws ER. Hajek and Hirsch: Otolaryngology pioneers of endonasal transsphenoidal pituitary surgery. Laryngoscope. 2023. 133: 807-13
2. Boetto J, Joitescu I, Raingeard I, Ng S, Le Corre M, Lonjon N. Endoscopic transsphenoidal surgery for non-functioning pituitary adenoma: Learning curve and surgical results in a prospective series during initial experience. Front Surg. 2022. 9: 959440
3. Conger A, Zhao F, Wang X, Eisenberg A, Griffiths C, Esposito F. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: Trends in repair failure and meningitis rates in 509 patients. J Neurosurg. 2019. 130: 861-75
4. Daly AF, Beckers A. The epidemiology of pituitary adenomas. Endocrinol Metab Clin North Am. 2020. 49: 347-55
5. Hanalioglu S, Gurses ME, Gecici NN, Baylarov B, Isikay I, Gürlek A. Repeat endoscopic endonasal transsphenoidal surgery for residual or recurrent Cushing’s disease: Safety, feasibility, and success. Pituitary. 2024. 27: 259-68
6. Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, Wayoff M. Endoscopic pituitary tumor surgery. Laryngoscope. 1992. 102: 198-202
7. Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P. Endoscopic endonasal skull base surgery: Analysis of complications in the authors’ initial 800 patients. J Neurosurg. 2011. 114: 1544-68
8. Khan N, Abboudi H, Khan MS, Dasgupta P, Ahmed K. Measuring the surgical ‘learning curve’: Methods, variables and competency. BJU Int. 2014. 113: 504-8
9. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993. 33: 610-7
10. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. The endoscope-assisted ventral approach compared with open microscope-assisted surgery for clival chordomas. World Neurosurg. 2011. 76: 318-27
11. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with anterior craniofacial and combined cranionasal resection of esthesioneuroblastomas. World Neurosurg. 2013. 80: 148-59
12. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg. 2012. 77: 329-41
13. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of giant pituitary adenomas. Pituitary. 2012. 15: 150-9
14. Koutourousiou M, Gardner PA, Tormenti MJ, Henry SL, Stefko ST, Kassam AB. Endoscopic endonasal approach for resection of cranial base chordomas: Outcomes and learning curve. Neurosurgery. 2012. 71: 614-24
15. Krisht KM, Sorour M, Cote M, Hardy J, Couldwell WT. Marching beyond the sella: Gerard Guiot and his contributions to neurosurgery. J Neurosurg. 2015. 122: 464-72
16. Kshettry VR, Do H, Elshazly K, Farrell CJ, Nyquist G, Rosen M. The learning curve in endoscopic endonasal resection of craniopharyngiomas. Neurosurg Focus. 2016. 41: E9
17. Little AS, Chapple K, Jahnke H, White WL. Comparative inpatient resource utilization for patients undergoing endoscopic or microscopic transsphenoidal surgery for pituitary lesions. J Neurosurg. 2014. 121: 84-90
18. Lofrese G, Vigo V, Rigante M, Grieco DL, Maresca M, Anile C. Learning curve of endoscopic pituitary surgery: Experience of a neurosurgery/ENT collaboration. J Clin Neurosci. 2018. 47: 299-303
19. Micko AS, Wöhrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: Endoscopic verification and its correlation with an MRI-based classification. J Neurosurg. 2015. 122: 803-11
20. Nakassa AC, Chabot JD, Snyderman CH, Wang EW, Gardner PA, Fernandez-Miranda JC. Complete endoscopic resection of a pituitary stalk epidermoid cyst using a combined infrasellar interpituitary and suprasellar endonasal approach: Case report. J Neurosurg. 2018. 128: 437-43
21. Nix P, Alavi SA, Tyagi A, Phillips N. Endoscopic repair of the anterior skull base-is there a learning curve?. Br J Neurosurg. 2018. 32: 407-11
22. O’Malley BW, Grady MS, Gabel BC, Cohen MA, Heuer GG, Pisapia J. Comparison of endoscopic and microscopic removal of pituitary adenomas: Single-surgeon experience and the learning curve. Neurosurg Focus. 2008. 25: E10
23. Patel KS, Komotar RJ, Szentirmai O, Moussazadeh N, Raper DM, Starke RM. Case-specific protocol to reduce cerebrospinal fluid leakage after endonasal endoscopic surgery. J Neurosurg. 2013. 119: 661-8
24. Pernar LI, Robertson FC, Tavakkoli A, Sheu EG, Brooks DC, Smink DS. An appraisal of the learning curve in robotic general surgery. Surg Endosc. 2017. 31: 4583-96
25. Prevedello DM, Doglietto F, Jane JA, Jagannathan J, Han J, Laws ER. History of endoscopic skull base surgery: Its evolution and current reality. J Neurosurg. 2007. 107: 206-13
26. Rychen J, Asmaro K, Constanzo F, Ljubimov VA, Lee MH, Rinaldi M. Endoscopic endonasal pituitary sacrifice for select tumors with retrochiasmatic and/or retrosellar extension: Surgical anatomy, operative technique, and case series. J Neurosurg. 2024. 141: 762-72
27. Schmidt RF, Choudhry OJ, Takkellapati R, Eloy JA, Couldwell WT, Liu JK. Hermann Schloffer and the origin of transsphenoidal pituitary surgery. Neurosurg Focus. 2012. 33: E5
28. Schwartz TH, Morgenstern PF, Anand VK. Lessons learned in the evolution of endoscopic skull base surgery. J Neurosurg. 2019. 130: 337-46
29. Younus I, Gerges MM, Uribe-Cardenas R, Morgenstern PF, Eljalby M, Tabaee A. How long is the tail end of the learning curve? Results from 1000 consecutive endoscopic endonasal skull base cases following the initial 200 cases. J Neurosurg. 2021. 134: 750-60