- Department of Neurosurgery, ASST Ovest Milanese, Legnano, Milano, Italy.
- Department of Neurosurgery, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy.
- Department of Neurosurgery, San Martino IST University Hospital, Genova, Italy.
Correspondence Address:
Giovanni Marco Sicuri, Department of Neurosurgery, ASST Ovest Milanese, Legnano, Milan, Italy.
DOI:10.25259/SNI_111_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Roberto Stefini1, Stefano Peron1, Alessandro La Camera2, Andrea Cividini1, Pietro Fiaschi3, Giovanni Marco Sicuri1. The positive effects of surgery on symptomatic stereotactic radiation-induced peritumoral brain edema: A report of three cases. 19-Jul-2021;12:358
How to cite this URL: Roberto Stefini1, Stefano Peron1, Alessandro La Camera2, Andrea Cividini1, Pietro Fiaschi3, Giovanni Marco Sicuri1. The positive effects of surgery on symptomatic stereotactic radiation-induced peritumoral brain edema: A report of three cases. 19-Jul-2021;12:358. Available from: https://surgicalneurologyint.com/surgicalint-articles/the-positive-effects-of-surgery-on-symptomatic-stereotactic-radiation-induced-peritumoral-brain-edema-a-report-of-three-cases/
Abstract
Background: Peritumoral brain edema is an uncommon but life-threatening side effect of brain tumors radiosurgery. Medical therapy usually alleviates symptoms until edema spontaneously disappears. However, when peritumoral brain edema endangers the patient’s life or medical therapy fails to guarantee an acceptable quality of life, surgery might be considered.
Case Description: Our report focuses on three patients who developed extensive peritumoral brain edema after radiosurgery. Two were affected by vestibular schwannomas and one by a skull-base meningioma. Peritumoral brain edema worsened despite maximal medical therapy in all cases; therefore, surgical removal of the radiated lesion was carried out. In the first patient, surgery was overdue and resulted in a fatal outcome. On the other hand, in the latter two cases surgery was quickly effective. In all three cases, an unmanageable brain swelling was not found at surgery.
Conclusion: Surgical removal of brain tumors previously treated with radiosurgery was safe and effective in resolving shortly peritumoral brain edema. This solution should be considered in patients who do not respond to medical therapy and before worsening of clinical conditions. Interestingly, the expected brain swelling was not confirmed intraoperatively. In our experience, this magnetic resonance finding should not be considered a criterion to delay surgery.
Keywords: Brain swelling, Meningioma, Peritumoral brain edema, Radiosurgery, Vestibular schwannoma
INTRODUCTION
The treatment strategy for newly diagnosed skull-based meningiomas and vestibular schwannomas provides several options including active surveillance, surgery, and radiosurgery. The best treatment strategy for asymptomatic or mildly symptomatic patients with small (<2 cm) or medium sized (2–3 cm) skull-base tumors is still debated. In meningiomas and vestibular schwannomas, radiosurgery offers a long-term local tumor control rate of 87–98% at 5–10 years.[
CLINICAL PRESENTATION
Case 1
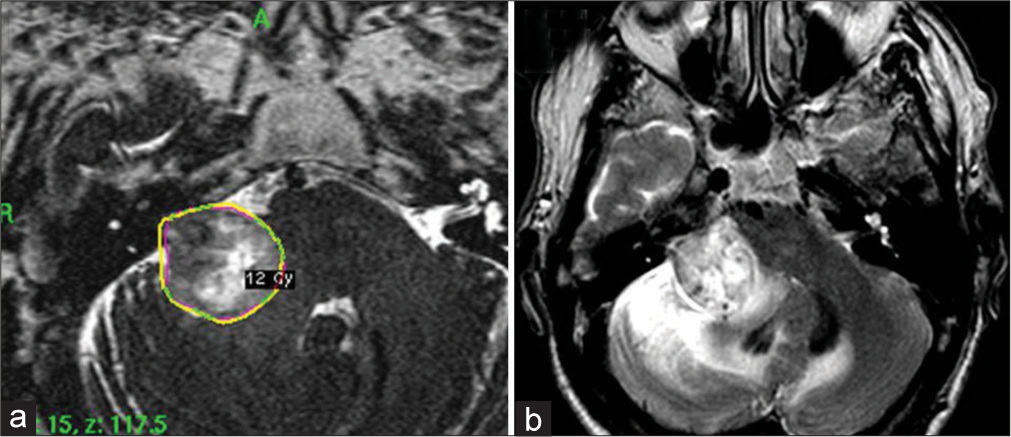
A 70-year-old man had been experiencing slowly-progressive hearing loss in the right ear for 3 years. A brain MRI revealed an acoustic neuroma in the right cerebellopontine angle (CPA), Koos Grade III, with peritumoral edema and brainstem compression [
Case 2
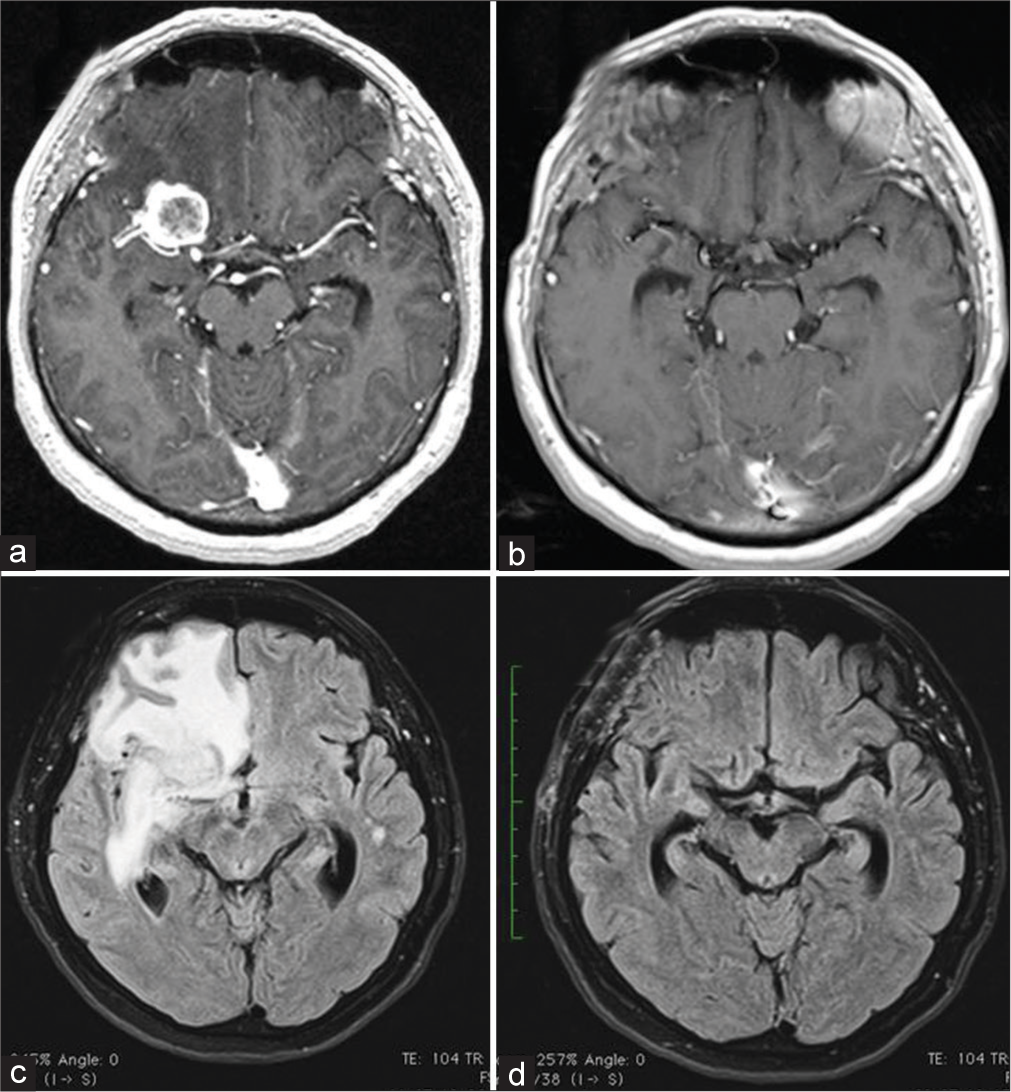
A 45-year-old woman with right-sided hearing loss underwent a brain MRI that showed a right CPA mass [
Figure 2:
Right vestibular schwannoma with maximum diameter of 41 × 35 × 35mm. MRI T1 contrast-enhanced image before first surgery (a). MRI T1 contrast-enhanced image of Gamma Knife Radiosurgery (GKR) treatment planning (b). MRI T2 image at 18 months after GKR (c); peritumoral brain edema (PTBE) in the right cerebellar hemisphere and brain stem is clearly visible. MRI T2 at image 3 months after surgical removal showing complete PTBE resolution (d).
Case 3
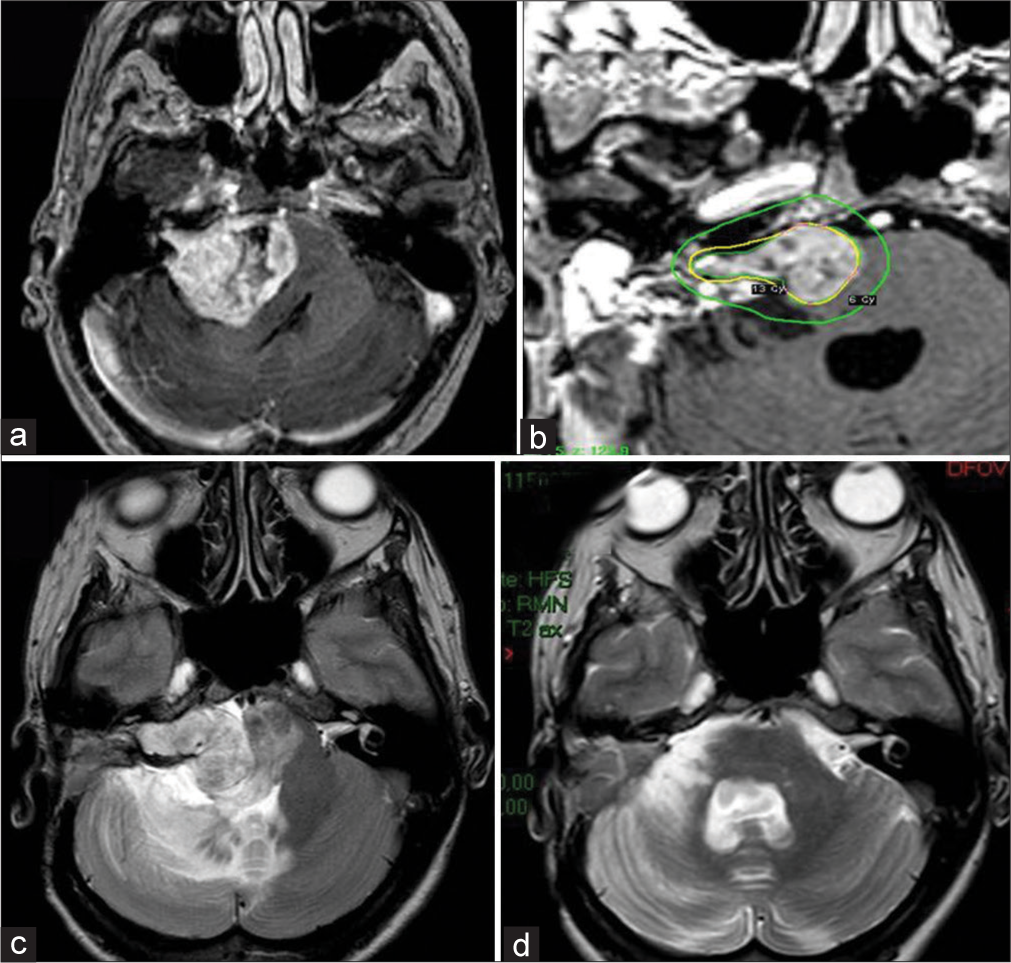
A 60-year-old woman had an incidental diagnosis of right clinoid meningioma. The lesion was about 2 cm diameter with moderate PTBE [
DISCUSSION
Herein, we present three cases of extensive PTBE after SRS where surgery was carried out after medical therapy had failed. All patients had some risk factors for developing PTBE. While symptomatic PTBE is normally treated with high-dose of steroids or osmotherapy, severe side effects such bowel perforation or Cushing disease, as described in Cases 1 and 3, respectively, must be considered. A relief effect of bevacizumab on edema induced by radiotherapy, mainly in malignant tumors, has been described.[
Moreover, the presence of massive PTBE did not imply dangerous intraoperative brain swelling in our series. Cerebral edema is sometimes thought of as synonymous of brain swelling that is quite true in neurotraumatology and in cerebral infarction. However, equally important are the biomechanical properties of the brain, such as its ability for elastic distortion. In radiated meningiomas hyperpermeability of blood vessels[
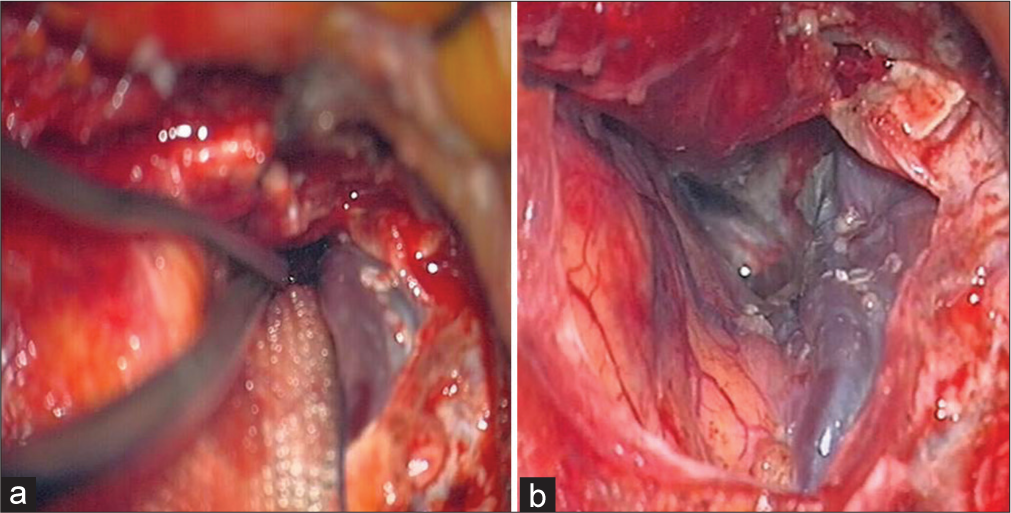
Regional impairment of venous drainage from the brain may also contribute to PTBE formation. Indeed, in case 3 a completely arterialized sylvian vein was noticed [
CONCLUSION
Although it is common knowledge that extensive PTBE complicates surgery and influences surgical outcome, prognosis, and risk of recurrence,[
In this setting indications for surgery are the presence of symptomatic PTBE induced by SRS, evident brain shift due to extensive PTBE, ineffective long-lasting medical treatment, and low surgical risks. However, decision-making must be individualized according to patient features, to the existence of a possible rapid evolving neurological worsening, and to the response to medical therapy and its side effects.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bhowmik A, Khan R, Ghosh MK. Blood brain barrier: A challenge for effectual therapy of brain tumors. Biomed Res Int. 2015. 2015: 320941
2. Budday S, Sommer G, Birkl C, Langkammer C, Haybaeck J, Kohnert J. Mechanical characterization of human brain tissue. Acta Biomater. 2017. 48: 319-40
3. Cai R, Barnett GH, Novak E, Chao ST, Suh JH. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery. 2010. 66: 513-22
4. Chang JH, Chang JW, Choi JY, Park YG, Chung SS. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry. 2003. 74: 226-30
5. El Shehaby A, Ganz JC, Reda WA, Hafez A. Mechanisms of edema after gamma knife surgery for meningiomas-report of two cases. J Neurosurg. 2005. 102: 1-3
6. Gilbert JJ, Paulseth JE, Coates RK, Malott D. Cerebral edema associated with meningiomas. Neurosurgery. 1983. 12: 599-605
7. Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: Evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013. 118: 557-65
8. Hasegawa T, Kida Y, Kobayashi T, Yoshimoto M, Mori Y, Yoshida J. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg. 2005. 102: 10-6
9. Hoe Y, Choi YJ, Kim JH, Kwon DH, Kim CJ, Cho YH. Peritumoral brain edema after stereotactic radiosurgery for asymptomatic intracranial meningiomas: Risks and pattern of evolution. J Korean Neurosurg Soc. 2015. 58: 379-84
10. Hossmann KA, Wechsler W, Wilmes F. Experimental peritumorous edema, Morphological and pathophysiological observations. Acta Neuropathol. 1979. 45: 195-203
11. Hou J, Kshettry VR, Selman WR, Bambakidis NC. Peritumoral brain edema in intracranial meningiomas: The emergence of vascular endothelial growth factor-directed therapy. Neurosurg Focus. 2013. 35: E2
12. Khan M, Zhao Z, Arooj S, Liao G. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: A systematic review and meta-analysis. BMC Cancer. 2021. 21: 167
13. Kobayashi T, Kida Y, Mori Y. Long-term results of stereotactic gamma radiosurgery of meningiomas. Surg Neurol. 2001. 55: 325-31
14. Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: Summary of experience in 829 cases. J Neurosurg. 2005. 102: 195-9
15. Mindermann T, Schlegel I. Grading of vestibular schwannomas and corresponding tumor volumes: Ramifications for radiosurgery. Acta Neurochir (Wien). 2013. 155: 71-4
16. Morgan TM, Zaenger D, Switchenko JM, Eaton BR, Crocker IR, Ali AN. Fractionated radiotherapy is associated with lower rates of treatment-related edema than stereotactic radiosurgery in magnetic resonance imaging-defined meningiomas. World Neurosurg. 2019. 121: e640-6
17. Novotný J, Kollová A, Liscák R. Prediction of intracranial edema after radiosurgery of meningiomas. J Neurosurg. 2006. 105: 120-6
18. Nunes FP, Merker VL, Jennings D, Caruso PA, di Tomaso E, Muzikansky A. Bevacizumab treatment for meningiomas in NF2: A retrospective analysis of 15 patients. PLoS One. 2013. 8: e59941
19. O’Connor KP, Algan O, Vesely SK, Palejwala AH, Briggs RG, Conner AK. Factors associated with treatment failure and radiosurgery-related edema in WHO grade 1 and 2 meningioma patients receiving gamma knife radiosurgery. World Neurosurg. 2019. 130: e558-65
20. Plotkin SR, Duda DG, Muzikansky A, Allen J, Blakeley J, Rosser T. Multicenter, prospective, Phase II and biomarker study of high-dose bevacizumab as induction therapy in patients with neurofibromatosis Type 2 and progressive vestibular schwannoma. J Clin Oncol. 2019. 37: 3446-54
21. ReMine SG, McIlrath DC. Bowel perforation in steroid-treated patients. Ann Surg. 1980. 192: 581-6
22. Salpietro FM, Alafaci C, Lucerna S, Iacopino DG, Todaro C, Tomasello F. Peritumoral edema in meningiomas: Microsurgical observations of different brain tumor interfaces related to computed tomography. Neurosurgery. 1994. 35: 638-41
23. Santacroce A, Walier M, Régis J, Liščák R, Motti E, Lindquist C. Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery. 2012. 70: 32-9
24. Sheehan JP, Lee CC, Xu Z, Przybylowski CJ, Melmer PD, Schlesinger D. Edema following Gamma knife radiosurgery for parasagittal and parafalcine meningiomas. J Neurosurg. 2015. 123: 1287-93
25. Shen G, Wang YJ, Guan YJ, Dong DP, Yang G, Li D. Relief effect of bevacizumab on severe edema induced by re-irradiation in brain tumor patients. Chin Med J (Engl). 2015. 128: 2126-9
26. Shirotani T, Shima K, Chigasaki H. Resolution of peritumoral brain edema following excision of meningioma. Acta Neurochir Suppl (Wien). 1994. 60: 416-8
27. Sindou M, Alaywan M. Role of pia-mater vascularization of the tumour in the surgical outcome of intracranial meningiomas. Acta Neurochir. 1994. 130: 90-3
28. Singh VP, Kansai S, Vaishya S, Julka PK, Mehta VS. Early complications following gamma knife radiosurgery for intracranial meningiomas. J Neurosurg. 2000. 93: 57-61
29. Stafford SL, Pollock BE, Foote RL, Link MJ, Gorman DA, Schomberg PJ. Meningioma radiosurgery: Tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001. 49: 1029-37
30. Unger KR, Lominska CE, Chanyasulkit J, Randolph-Jackson P, White RL, Aulisi E. Risk factors for posttreatment edema in patients treated with stereotactic radiosurgery for meningiomas. Neurosurgery. 2012. 70: 639-45
31. Yamasaki F, Kolakshyapati M, Takano M, Yonezawa U, Nishibuchi I, Imano N. Effect of bevacizumab against cystic components of brain tumors. Cancer Med. 2019. 8: 6519-27
32. Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: Mechanism, efficacy and issues. Mol Cancer. 2019. 18: 21