- Department of Neurosurgery, Hospital de San José - Fundación Universitaria de Ciencias de la Salud, Bogota, Colombia,
- Department of Neurosurgery, Mayo Clinic, Jacksonville, Florida, USA,
- Department of Neurosurgery, Faculty of Medicine, Medical University of Plovdiv, Plovdiv, Bulgaria,
- Department of Neurosurgery, School of Medicine, Hospital de San José - Fundación Universitaria de Ciencias de la Salud, Bogota, Colombia.
Correspondence Address:
Edgar G. Ordóñez-Rubiano Department of Neurosurgery, Hospital de San José - Fundación Universitaria de Ciencias de la Salud (FUCS), Bogota, Colombia.
DOI:10.25259/SNI_59_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Edgar G. Ordóñez-Rubiano1, Luisa F. Figueredo2, Carlos A. Gamboa-Oñate1, Ivo Kehayov3, Jorge A. Rengifo-Hipus4, Ingrid J. Romero-Castillo4, Angie P. Rodríguez-Medina4, Javier G. Patiño-Gomez1, Oscar Zorro1. The reverse question mark and L.G. Kempe incisions for decompressive craniectomy: A case series and narrative review of the literature. 08-Jul-2022;13:295
How to cite this URL: Edgar G. Ordóñez-Rubiano1, Luisa F. Figueredo2, Carlos A. Gamboa-Oñate1, Ivo Kehayov3, Jorge A. Rengifo-Hipus4, Ingrid J. Romero-Castillo4, Angie P. Rodríguez-Medina4, Javier G. Patiño-Gomez1, Oscar Zorro1. The reverse question mark and L.G. Kempe incisions for decompressive craniectomy: A case series and narrative review of the literature. 08-Jul-2022;13:295. Available from: https://surgicalneurologyint.com/surgicalint-articles/11704/
Abstract
Background: Decompressive craniectomy (DC) is a lifesaving procedure, relieving intracranial hypertension. Conventionally, DCs are performed by a reverse question mark (RQM) incision. However, the use of the L. G. Kempe’s (LGK) incision has increased in the last decade. We aim to describe the surgical nuances of the LGK and the standard RQM incisions to treat patients with severe traumatic brain injury (TBI), intracranial hemorrhage (ICH), empyema, and malignant ischemic stroke. Furthermore, to describe, surgical limitations, wound healing, and neurological outcomes related to each technique.
Methods: To describe a prospective acquired, case series including patients who underwent a DC using either an RQM or an LGK incision in our institution between 2019 and 2020.
Results: A total of 27 patients underwent DC. Of those, ten patients were enrolled. The mean age was 42.1 years (26–71), and 60% were male. Five patients underwent DC using a large RQM incision; three had severe TBI, one ICH, and one ischemic stroke. The other five patients underwent DC using an LGK incision (one ICH, one subdural empyema, and one ischemic stroke). About 50% of patients presented severe headaches associated with vomiting, and six presented altered mental status (drowsy or stuporous). Motor deficits were present in four cases. In patients with ischemic or hemorrhagic stroke, symptoms were directly related to the stroke location. Hospital stays varied between 13 and 22 days. No readmissions were recorded, and no fatal outcome was documented during the follow-up.
Conclusion: The utility of the LGK incision is comparable with the classic RQM incision to treat acute brain injuries, where an urgent decompression must be performed. Some of these cases include malignant ischemic strokes, ICH, and empyema. No differences were observed between both techniques in terms of prevention of scalp necrosis and general cosmetic outcomes.
Keywords: Decompressive craniectomy, Intracranial hemorrhage, Kempe, Traumatic brain injury
INTRODUCTION
Brain edema results from a multifactorial combination of pathological mechanisms that vary depending on the etiology of the brain injury.[
In wartime, neurosurgeons have noted a breakdown of the RQM incision along the posterior curve. The posterior portion of the scalp flap is subject to dependent swelling and more surface contact.[
The DC was first reported by Kocher and Cushing[
MATERIALS AND METHODS
Clinical data and study design
This is a retrospective case series study. Patients who underwent a DC using either an LGK incision or a large RQM incision were enrolled. Patients were admitted to the emergency department in our institution between January 2019 and January 2020. Inclusion criteria included patients over 18 year old, with severe TBI, ICH, or subdural empyema with radiological signs of brain edema and evident clinical findings of intracranial hypertension. Radiological findings, including midline shift and subtle or noticeable signs of mass effect, including effacement of the ipsilateral lateral ventricle or effacement of the basal cisterns, were used to enroll patients into the study. Exclusion criteria included patients who underwent large craniotomies for brain tumor surgery and those with a severe TBI that underwent DC, where information was not complete for adequate characterization, or when the technical surgical features were not achieved, including a wide exposure in cases of trauma, where the first goal was the surgical draining of a subdural hematoma or where the primary goal of surgery was not treating mainly intracranial hypertension due to brain edema (e.g., epidural hematoma). Authorization was requested to our Institutional Ethics Board to include the information of the subjects in this study, preserving their identity both in the analysis of the information and in all images presented. This research was performed following the Declaration of Helsinki. This is a retrospectively analyzed study with approval by our Institutional Review Board.
Surgical procedure
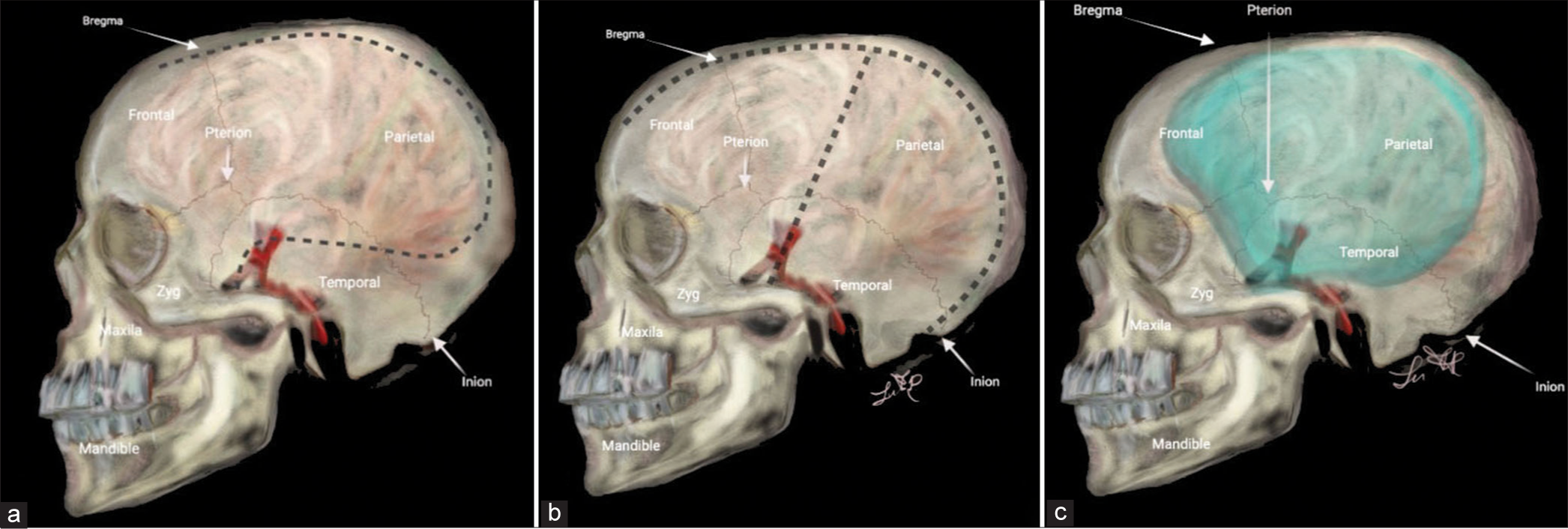
All patients underwent a nonenhanced head CT scan, and those with ICH or subdural empyema underwent an enhanced CT scan whenever possible. A contralateral external ventricular drain (EVD) was placed in the same procedure, except for patients with subdural empyema. The patients were positioned supine, lateral-sided opposite to DC, with the head rotated 45° for adequate hemispheric exposure. For the Kempe’s incision, the scalp was incised overlying the sagittal suture from the widow’s peak to the inion with a “T-bar” extension. The incision was started 1–2 cm anterior to the tragus at the temporal root of the zygoma and extending superiorly to meet the midline sagittal incision approximately 1 cm behind the coronal suture [
On the other hand, the RQM scalp incision started 1 cm anterior to the tragus at the root of the zygoma, curving posteriorly above and behind the ear toward the asterion. The incision is gently curved around the parietal bone to the midline and directed to the widow’s peak.[
RESULTS
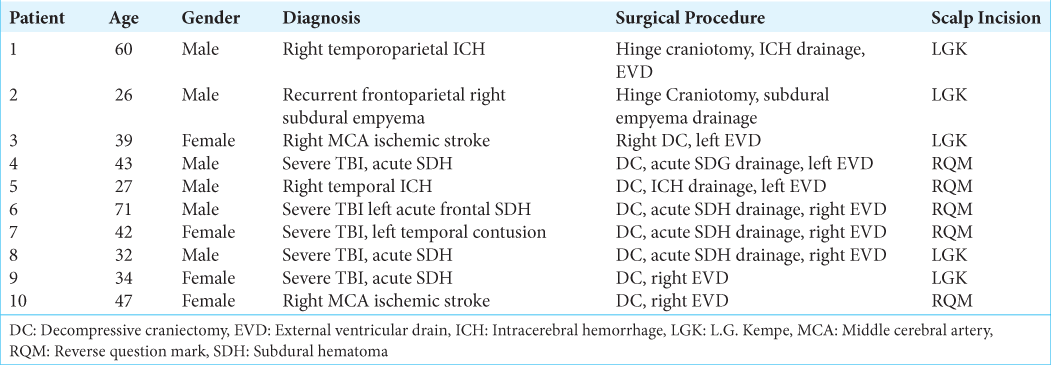
A total of 27 patients underwent DC. Of those, ten patients met inclusion criteria and were enrolled, accordingly. The mean age was 42.1 years (26–71), and 50% were male. Five patients underwent DC using a large RQM incision; three had severe TBI, one ICH, and one ischemic stroke. The other five patients underwent DC using an LGK incision (two with severe TBI, one ICH, one subdural empyema, and one ischemic stroke) [
ILLUSTRATIVE CASES
Case 1 – Intracranial hemorrhage
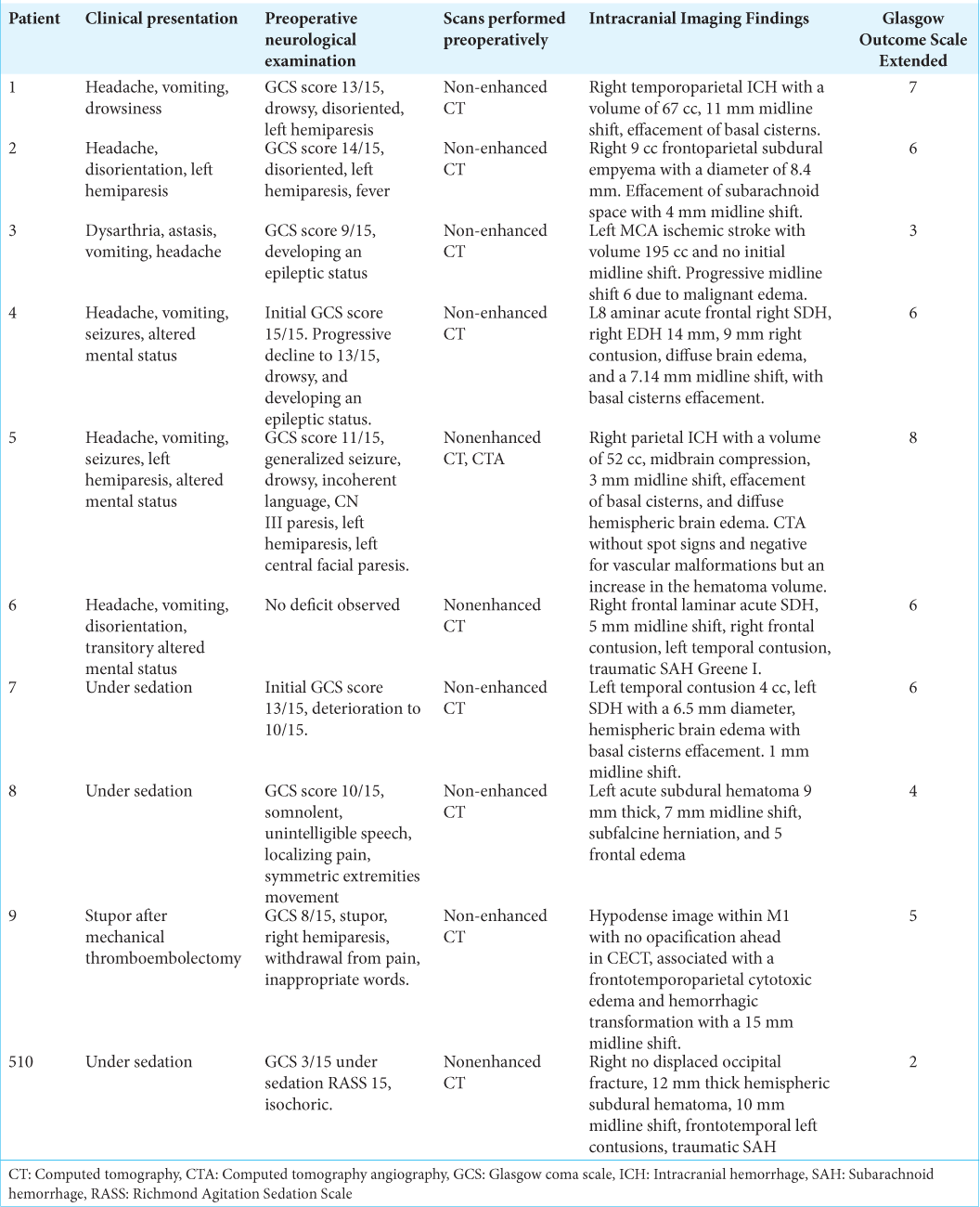
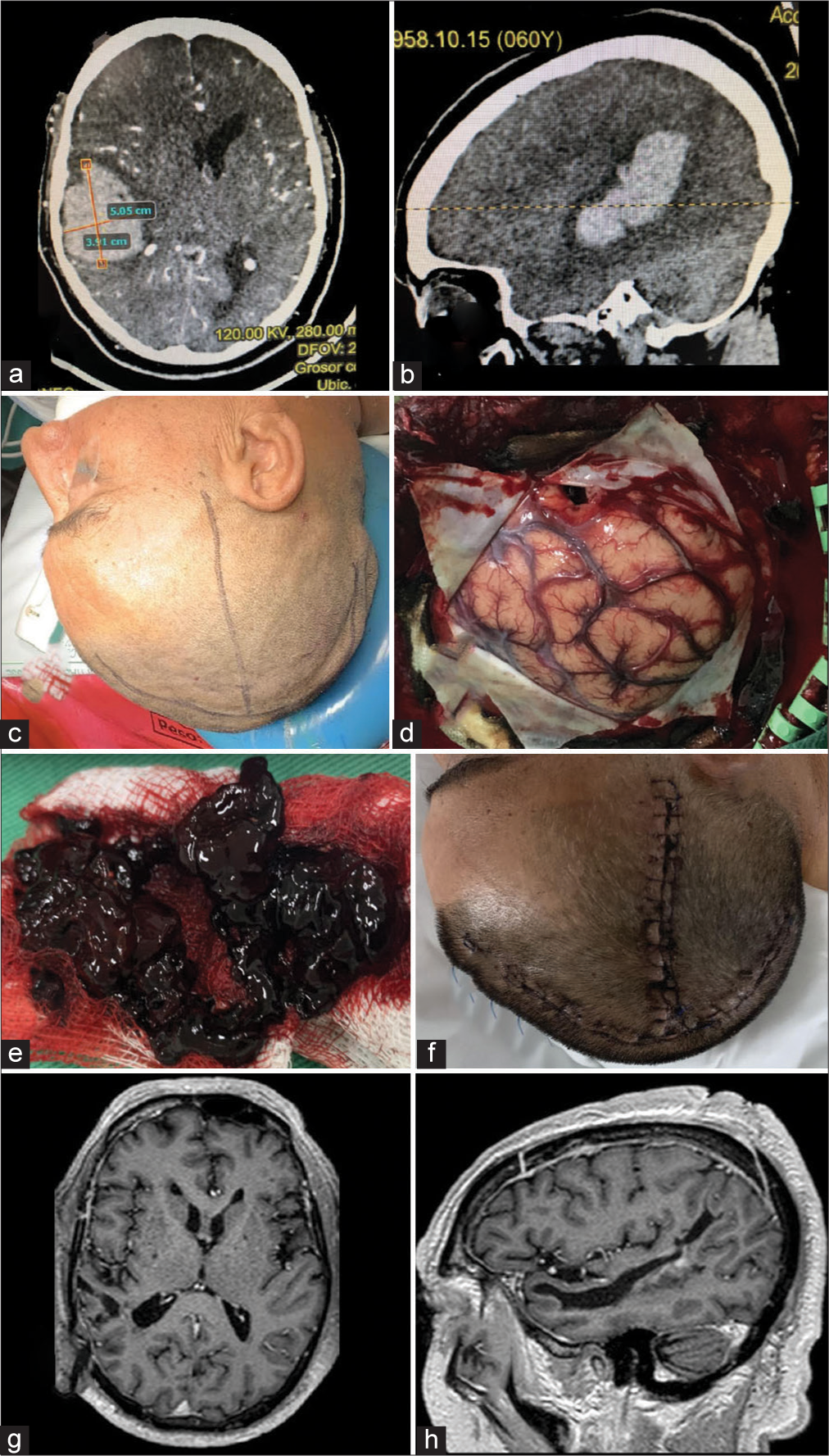
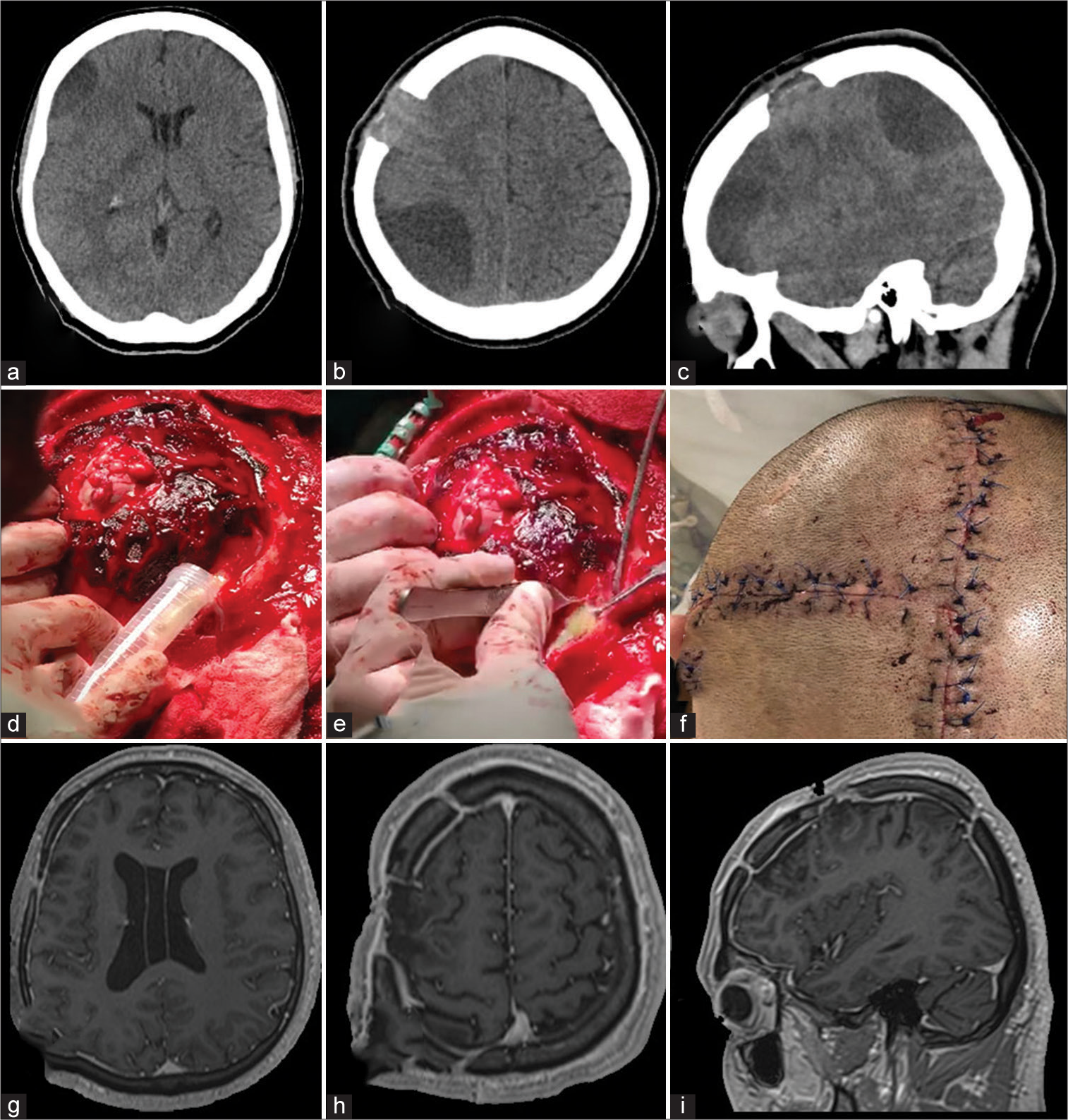
A 60-year-old male presented to the ED with a sudden loss of consciousness while drinking alcohol with his wife. The patient had a history of moderate alcohol consumption and nontreated hypertension. On his physical examination, he presented with a Glasgow Coma Scale (GCS) score of 13/15. He was drowsy, disoriented, and with left hemiparesis. The nonenhanced CT scan demonstrated a right temporoparietal ICH with significant mass effect and midline shift to the left [
Figure 2:
Intracranial Hemorrhage. (a and b) Preoperative enhanced CT scan of the head demonstrating a large intraparenchymal hemorrhage with significant mass effect and secondary midline shift to the left. No extravasation after contrast administration was noticed. (c) Preoperative scalp marking (d) brain exposure. A small corticectomy in the most basal aspect of the brain exposure is demonstrated. (e) The drained clot counted for approximately 5 cm3 volume is observed. (f) Postoperative picture of the inverted T-bar incision without evidence of dehiscence. (g and h) 1-year follow-up postoperative post contrast axial and sagittal T1 images.
Case 2 – Subdural empyema
A 26-year-old male presented with an acute onset headache, confusion, and left hemiparesis after a suicidal attempt with coumarin (rat poison) consumption. The patient underwent drainage for a right subdural empyema through a right frontal burr hole. On postoperative day 3, the patient presented consciousness impairment with acute decline to a GCS score 7/15. A recurrent right 8.4 mm frontoparietal subdural empyema with a 4 mm midline shift to the left was detected in the postoperative nonenhanced CT scan [
Figure 3:
Subdural empyema. (a-c) Non-enhanced CT scan demonstrates a recurrent subdural empyema with separate extension to the right frontal and parietal lobes. (d-e) Intraoperative images show the purulent collection drainage, remarkable brain edema, and epidural bleeding. (f) A postoperative picture of the T-bar incision is demonstrated. (g-i) One-year follow-up enhanced MRI of the head shows no recurrent empyema. (g-h) One-year postoperative MRI with gadolinium administration showed encephalomalacia with the recovery of the normal position of the frontal and temporal lobes.
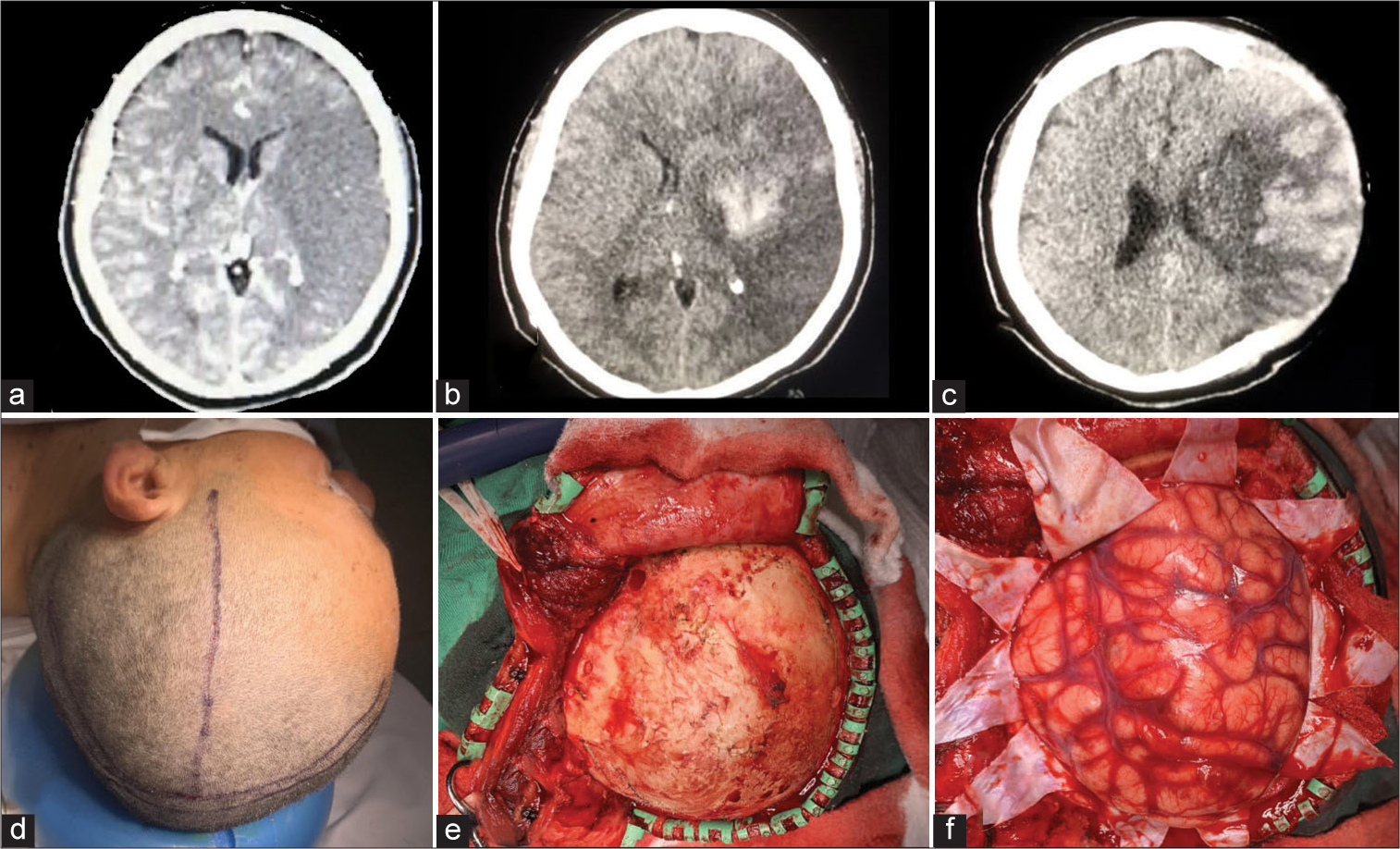
Case 8 – Traumatic brain injury
A 32-year-old man presented to the emergency department after a car accident. No relevant clinical antecedents were present. The patient presented under sedation and on his physical examination presented a GCS score of 8/15, with a Richmond Agitation Sedation Scale of −3. Brainstem reflexes were present, and the left pupil was 5 mm and fixed. The CT scan revealed a left acute subdural hematoma with a midline shift to the right and effacement of the basal cisterns [
Figure 4:
L.G. Kempe’s incision for trauma. (a and b) A preoperative CT scan demonstrates a left acute subdural hematoma with mass effect and a midline shift to the right with effacement of the basal cisterns. (c-f) An inverted “T-bar” incision is demonstrated, with consequent drainage of the hematoma and duroplasty. (g-h) Postoperative CT scan shows complete drainage of the hematoma, recovery of the midline, and the cranial defect in the left parietotemporal cranial vault.
Case 10 – Ischemic stroke
A 39-year-old woman presented with acute onset of dysarthria, ataxia, emesis, and headaches. She developed a status epilepticus after hospital admission. The CT scan showed a large left middle cerebral artery ischemic stroke [
Figure 5:
Decompressive craniectomy for ischemic stroke. (a) Admission-enhanced CT scan of the head demonstrates a large left middle cerebral artery infarction. (b) Two-hour admission nonenhanced CT scan shows hemorrhagic transformation of the stroke with significant edema and midline shift to the right. (c) Postoperative CT scan demonstrates recovery of the midline and transcranial herniation of the infarcted parenchyma. (d) Skin marking with a T-shaped Kempe’s mark. (e) Bone exposure. In this picture, the inferior displacement of the temporal muscle allows adequate exposure of the cranium. (f) After opening the dura mater, significant edema was evident.
DISCUSSION
Our study describes additional applications for the LGK incision. Some of the pathologies include empyema, intracranial hemorrhage, and ischemic strokes, considering the common pathophysiologic background of all of them: an increased ICP. Both techniques, RQM and LGK incisions, could be performed similarly to treat different entities. It is essential to consider that neurological prognosis depends directly on the severity and location of the injury. The direct decrease in ICP may improve overall survival for all pathologies in the same way that it has been described for TBI previously.[
CD has been previously used for ischemic strokes.[
A different indication for decompression is the subdural empyema. Subdural empyema is a collection of purulent material between the dura and the arachnoid. It is a life-threatening entity that is generally associated with paranasal sinusitis, otitis media, or mastoiditis.[
In the setting of epilepsy, the requirement for extensive hemispherectomies makes mandatory the use of a wide craniotomy. An example of this is an old work published in 1995 by Peacock et al. In that work, all cases were performed using an LGK incision, having successful results; however, they did not compare the incision with other techniques. They reported that only three patients had a mild neuro infection, which was easily controlled with antibiotics.[
Conventionally, DC has been widely used for patients with intracranial hypertension after severe DTI and most published series have performed RQM for this purpose. L.G. Kempe made the first description of a “T-shaped” incision.[
In regard to cosmetic outcomes, in 2020, Safari et al. evaluated the differences between both incision techniques for large craniotomies. In this study, 23 patients were followed for 6 months postoperatively. Their evaluation included aesthetic aspects assessed by the Stony Brook Scar Evaluation Scale (SBSES), which describes a qualitative subjective perspective from the patients about their scars. Based on the postoperative hair follicle density changes, either group showed any difference (P = 0.657), and the difference between the SBSES significantly favored the T-shaped incision (P = 0.005).[
The literature is variable in regard of surgical complications. An old problem with the Kempe’s incision is the necrosis in the intersections of the T-section.[
Limitations
The availability of information related to the LGK incision for other pathologies rather than TBI increases the bias at the time of objective evaluation. However, in the different settings reported, including bilateral lesions, empyema, malignant strokes, intracranial hemorrhages, and even in cosmetic outcomes, the T-shaped incision exceeded the expectations over the classic RQM. This study did not evaluate the times needed to complete the craniectomy neither for closure. However, it seems that LGK incision could be faster to complete the decompression but longer for skin closure. Certainly, further studies are required to scrutinize potential uses and differences between both techniques.
CONCLUSION
The utility of LGK T-shaped incision seems to be comparable with the traditional RQM incision to treat traumatic injuries and other pathologies, where an urgent decompression should be performed, including malignant ischemic strokes, ICH, and empyema. In terms of prevention of scalp necrosis and general cosmetic outcomes, no differences were found. Finally, the decision whether to use either technique should be performed based on a case-by-case manner as well as on the neurosurgeon’s preference and experience to provide the best option for each patient.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agrawal A, Timothy J, Pandit L, Shetty L, Shetty J. A review of subdural empyema and its management. Infect Dis Clin Pract. 2007. 15: 149-53
2. Becerra JM, Reis RS, Frank LD, Ramirez-Marrero FA, Welle B, Arriaga Cordero E. Transport and health: A look at three Latin American cities. Cad Saude Publica. 2013. 29: 654-66
3. Bor-Seng-Shu E, Figueiredo EG, Amorim RL, Teixeira MJ, Valbuza JS, de Oliveira MM. Decompressive craniectomy: A meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J Neurosurg. 2012. 117: 589-96
4. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ.editors. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017. 80: 6-15
5. Cushing H. I. Subtemporal decompressive operations for the intracranial complications associated with bursting fractures of the skull. Ann Surg. 1908. 47: 641-644.1
6. Dunn LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002. 73: i23-7
7. Eberle BM, Schnüriger B, Inaba K, Gruen JP, Demetriades D, Belzberg H. Decompressive craniectomy: Surgical control of traumatic intracranial hypertension may improve outcome. Injury. 2010. 41: 894-8
8. Hossain-Ibrahim M, Tarnaris A, Wasserberg J. Decompressive craniectomy friend or foe?. Trauma. 2012. 14: 16-38
9. Kempe LG. Hemispherectomy. Oper Neurosurg. 1968. 1: 179-89
10. Kobrine AI, Kempe LG. Studies in head injury. I. An experimental model of closed head injury. Surg Neurol. 1973. 1: 34-7
11. Kobrine AI, Kempe LG. Studies in head injury. II. Effect of dexamethasone on traumatic brain swelling. Surg Neurol. 1973. 1: 38-42
12. Lee OF, Patiño-Ladino S. Decompressive craniectomy for empyema by Aggregatibacter aphrophilus Literature rivew and case report. Univ Med. 2019. 60: 86-95
13. Moscote-Salazar LR, Alvis-Miranda HRL, Ramos-Villegas Y, Quintana-Pajaro L, Rubiano AM, Alcalá-Cerra G. Refractory traumatic intracranial hypertension: The role of decompressive craniectomy. Cir Cir. 2019. 87: 358-64
14. Nathoo N, Nadvi SS, Gouws E, van Dellen JR. Craniotomy improves outcomes for cranial subdural empyemas: Computed tomography-era experience with 699 patients. Neurosurgery. 2001. 49: 872-7
15. Pallesen LP, Barlinn K, Puetz V. Role of decompressive craniectomy in ischemic stroke. Front Neurol. 2018. 9: 1119
16. Peacock WJ, Wehby-Grant MC, Shields WD, Shewmon DA, Chugani HT, Sankar R. Hemispherectomy for intractable seizures in children: A report of 58 cases. Childs Nerv Syst. 1996. 12: 376-84
17. Ragel BT, Klimo P, Martin JE, Teff RJ, Bakken HE, Armonda RA. Wartime decompressive craniectomy: Technique and lessons learned. Neurosurg Focus. 2010. 28: E2
18. Rubiano AM, Maldonado M, Montenegro J, Restrepo CM, Khan AA, Monteiro R. The evolving concept of damage control in neurotrauma: Application of military protocols in civilian settings with limited resources. World Neurosurg. 2019. 125: e82-93
19. Safari H, Bagher S, Halili BA. Cosmetic outcomes of scalp in standard reverse question mark incision and L.G. Kempe incision in large craniotomies. Iran J Neurosurg. 2020. 6: 203-10
20. Schirmer CM, Ackil AA, Malek AM. Decompressive craniectomy. Neurocrit Care. 2008. 8: 456-70
21. Veldeman M, Daleiden L, Hamou H, Höllig A, Clusmann H. An altered posterior question-mark incision is associated with a reduced infection rate of cranioplasty after decompressive hemicraniectomy. J Neurosurg. 2020. 134: 1262-70
22. Wada Y, Kubo T, Asano T, Senda N, Isono M, Kobayashi H. Fulminant subdural empyema treated with a wide decompressive craniectomy and continuous irrigation case report. Neurol Med Chir (Tokyo). 2002. 42: 414-6
23. Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am. 2002. 13: 371-83







Dr Ahmed Bakhsh
Posted January 10, 2023, 11:17 pm
an excellant article
This new technique is better than an old one