- Department of Neurosurgery, Chubu Medical Center for Prolonged Traumatic Brain Dysfunction, Minokamo, Japan
- Department of Neurosurgery, Daiyukai General Hospital, Ichinomiya, Japan
- Department of Neurosurgery, National Hospital Organization Toyohashi Medical Center, Toyohashi, Japan.
Correspondence Address:
Naoya Imai, Department of Neurosurgery, Chubu Medical Center for Prolonged Traumatic Brain Dysfunction, Minokamo, Japan.
DOI:10.25259/SNI_293_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Naoya Imai1, Takayuki Kato2, Yohei Ito3, Ryo Morishima2, Tatsuki Aki2, Shin-ichi Shirakami2. Timing of chronic subdural hematoma treatment affects middle meningeal artery embolization outcome. 21-Jun-2024;15:214
How to cite this URL: Naoya Imai1, Takayuki Kato2, Yohei Ito3, Ryo Morishima2, Tatsuki Aki2, Shin-ichi Shirakami2. Timing of chronic subdural hematoma treatment affects middle meningeal artery embolization outcome. 21-Jun-2024;15:214. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12948
Abstract
Background: Chronic subdural hematoma (CSDH) is a condition that tends to recur frequently. Although middle meningeal artery embolization (MMAE) is an effective CSDH treatment, there is currently no consensus regarding the optimal timing for embolization.
Methods: In this single-center and retrospective study, we reviewed 72 cases with 1st-time recurrent CSDH from January 2018 to July 2023 and identified those treated with MMAE to examine its effect and the impact of differences in the timing of treatment.
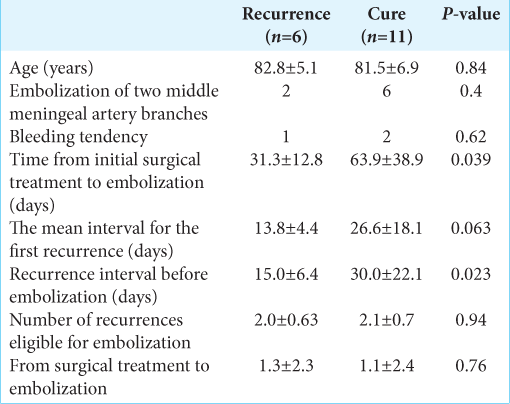
Results: Of the 72 cases with CSDH recurrence for the 1st time (mean age: 80.4 ± 9.7 years; men: 62 [86.1%]; mean first recurrence interval: 33 ± 24 days), 27 (37.5%) experienced a second recurrence. The mean first recurrence interval was shorter in cases with a second recurrence compared to cured cases: 24.3 ± 18.6 versus 38.3 ± 25.6 days, respectively (P = 0.005). MMAE was performed in 17 (23.6%) cases (mean age: 82 ± 6.2 years; men: 14 [82.4%]). The mean time from initial surgical treatment to embolization was 52.4 ± 35.4 days, and the mean recurrence interval before MMAE was 24.9 ± 19.6 days. Six cases (35.3%) experienced post-embolization recurrence and required surgical treatment. The mean recurrence interval before MMAE was shorter in cases with recurrence after MMAE (15 ± 6.4 vs. 30 ± 22.1 days, P = 0.023). The time from initial surgical treatment to embolization was significantly shorter: 31.3 ± 12.8 versus 63.9 ± 38.9 days (P = 0.039).
Conclusion: Cases with a short first recurrence interval were more likely to experience a second recurrence. Repeated recurrences within a short time increased the likelihood of post-embolization recurrence. MMAE performed early following the initial surgical treatment increased the recurrence risk.
Keywords: Chronic subdural hematoma, Embolization, Endovascular therapy, Recurrence
INTRODUCTION
Chronic subdural hematoma (CSDH) is a prevalent neurosurgical condition characterized by symptoms resulting from the gradual accumulation of blood in the subdural space, which compresses the brain. There are two main forms of CSDH: traumatic and spontaneous. Traumatic CSDH is triggered by trauma that could sometimes be so minor that the individual is either unaware of its occurrence or may have forgotten it. CSDH that is not linked to any underlying trauma and without a known cause is referred to as spontaneous. Examples of spontaneous CSDH include patients with increased bleeding tendency and those with cancer, but many aspects of the disease remain unknown. As the severity of symptoms increases, surgical intervention, such as burr-hole drainage of the hematoma, may be necessary; however, the likelihood of recurrence is high. CSDH often occurs in older adults, and it is anticipated that the number of cases will increase as the population ages.[
In this single-center and retrospective study, we examined the recurrence interval and treatment course of cases with recurrent CSDH and discussed the postoperative course of MMAE and the timing of embolization.
MATERIALS AND METHODS
This study is a single-center and retrospective analysis of 72 lesions of 1st-time recurrent symptomatic CSDH that occurred between January 2018 and July 2023. We examined the case background, number of recurrences, recurrence interval (the period between surgical treatments), and treatment outcomes for each patient. Further, we retrospectively reviewed the chronological sequence from initial onset to cure, embolization method, and treatment outcomes of patients who underwent MMAE. The Institutional Ethics Review Board approved the study (approval number: 2023-013). The timing and methods of treatment at our center are as follows: symptomatic CSDH was initially treated using burr-hole evacuation or drainage. If the hematoma regrew and became symptomatic again, further surgical treatment was performed. Surgical treatment included mini craniotomy and endoscopic removal of the hematoma, particularly for organized hematomas or those that could not be removed through burr holes.
MMAE was usually performed at the second recurrence, making it the third surgical treatment. At other times, MMAE was performed when there was a high risk of recurrence or contralateral symptomatic disease at the time of embolization. The embolization material used was n-butyl-2-cyanoacrylate. Proximal and distal branch embolization was performed if dangerous anastomoses were absent and catheter guidance was possible; otherwise, proximal or distal branch embolization was performed.
Continuous variables are reported as means and standard deviations, whereas categorical variables are reported as numbers and frequencies (percentages). EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) was used for all statistical analyses. The Mann–Whitney U-test was used for continuous variables, and Pearson’s Chi-square test was used for categorical variables. Statistical significance was set at P < 0.05.[
RESULTS
Seventy-two cases with a second recurrence were included in the study. No cases were excluded from the study. The mean age of participants was 80.4 ± 9.7 years; 62 (86.1%) were men, and 28 (38.9%) recurrences were right-sided. The mean first recurrence interval was 33 ± 24 days, and the mean of all recurrence intervals was 31.9 ± 26.3 days. Bleeding tendency (hematological disease or oral antithrombotic medication) was present in 25 cases (34.7%) [
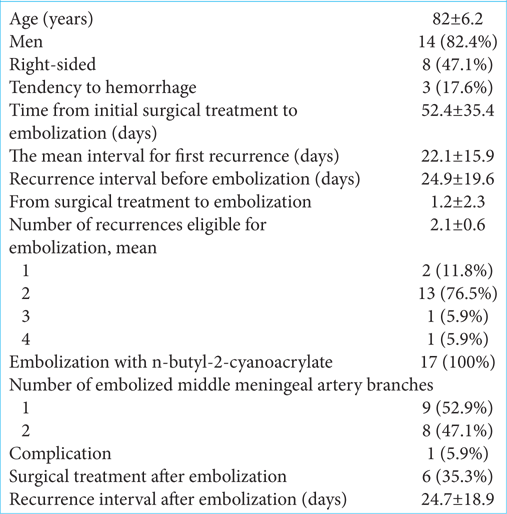
MMAE was performed in 17 (23.6%) cases (mean age: 82 ± 6.2 years; men: 14 [82.4%]), and 8 (47.1%) were right-sided. The mean first recurrence interval was 22.1 ± 15.9 days, the mean time from initial surgical treatment to embolization was 52.4 ± 35.4 days, and the mean recurrence interval before embolization was 24.9 ± 19.6 days. Embolization was performed 2 days before and 6 days after surgical treatment, with a mean of 1.2 ± 2.3 days after surgery. Embolization was performed in 2 cases (11.8%) at the first recurrence, 13 (76.5%) at the second, 1 (5.9%) at the third, and 1 (5.9%) at the fourth recurrence (mean: 2.1 recurrences). Three cases had an increased bleeding tendency, two were receiving antithrombotic medication, and one had myelodysplastic syndrome [
All cases underwent successful embolization using n-butyl-2-cyanoacrylate (20–33%); nine cases underwent embolization of one branch of the MMA, eight underwent embolization of two branches, and one had a complicated middle meningeal arteriovenous shunt. After embolization, 6 cases (35.3%) became symptomatic and required surgical treatment [
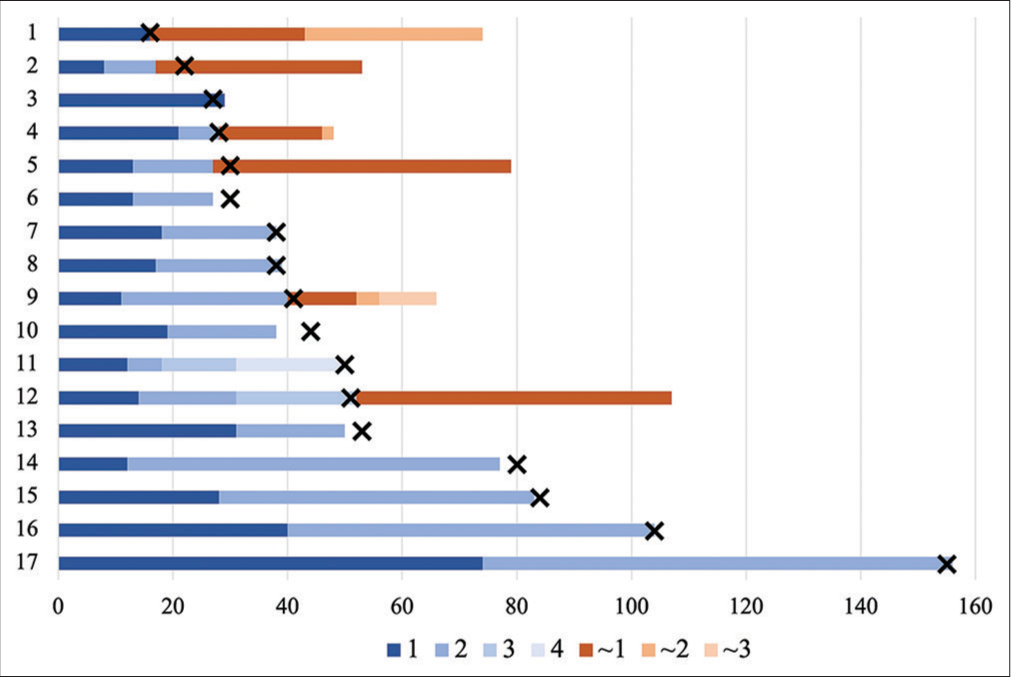
Figure 2:
The treatment course progress of middle meningeal artery embolized cases, including the embolization date, in order of the number of days from initial surgical treatment to embolization. The blue bar indicates the recurrence interval of pre-middle meningeal artery embolization (MMAE). × indicates the timing of embolization. Red indicates the recurrence interval after MMAE.
In cases that required surgical treatment after embolization compared to those that were cured, the mean first recurrence interval was 13.8 ± 4.4 versus 26.6 ± 18.1 days, respectively (P = 0.63). The mean interval for all recurrences before MMAE was shorter in cases that required surgical treatment after MMAE compared to those not requiring surgery (15 ± 6.4 vs 30 ± 22.1 days, respectively; P = 0.023). The time from initial surgical treatment to embolization was also significantly shorter (31.3 ± 12.8 vs. 63.9 ± 38.9 days; P = 0.039;
DISCUSSION
Older age, antithrombotic medications, pre-and postoperative hematoma status, and volume are known risk factors for CSDH recurrence. Although there are reports that drainage does not affect the time to recurrence,[
Several studies have documented the efficacy of MMAE for CSDH. Although various reports have focused on whether embolization should be performed as a standalone treatment or in conjunction with surgical treatment, the type of embolization material to be used, the number of vessels to be embolized,[
CSDH formation involves the recruitment of inflammatory cells, angiogenesis of highly permeable and leaky capillaries, processes supporting membrane formation, and fibrinolysis, which promote further bleeding.[
Embolization of the MMA is believed to be due to its hemostatic effect and inflammatory cascade arrest. MMA embolization alone reportedly reduces the hematoma after 3–12 weeks.[
The potential for bleeding, including the use of antithrombotic therapy, is an important factor in the recurrence of CSDH. Although reports are indicating that antithrombotic therapy increases the likelihood of CSDH recurrence and the recurrence after MMAE, particularly in spontaneous cases,[
The first recurrence interval was associated with the second recurrence of CSDH but not with the recurrence after MMAE. In cases with a short interval to the first recurrence, MMAE may reduce the number of recurrences and extend the recurrence interval. However, it should be noted that recurrence after MMAE may occur if the time between initial treatment and embolization is short.
A limitation of this study was the high recurrence rate after embolization. Previous meta-analyses have reported a 3–5% likelihood of surgical treatment following MMA treatment.[
CONCLUSION
Cases with a short first recurrence interval were more likely to experience a second CSDH recurrence. Cases with repeated recurrences within a short time were more likely to experience recurrence after embolization. Cases in which MMAE was performed early after the initial surgical treatment were prone to recurrence. The treatment timing is also an important factor impacting recurrence rates and should be carefully considered. Prospective studies and accumulating cases are required, with a special focus on the timeline, including recurrence interval and treatment timing.
Ethical approval
The research/study was approved by the Institutional Review Board at Daiyukai General Hospital, number 2023–013, dated September 06, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adhiyaman V, Chattopadhyay I, Irshad F, Curran D, Abraham S. Increasing incidence of chronic subdural haematoma in the elderly. QJM. 2017. 110: 375-8
2. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017. 14: 108
3. Housley SB, Monteiro A, Khawar WI, Donnelly BM, Lian MX, Fritz AG. Volumetric resolution of chronic subdural hematomas treated with surgical evacuation versus middle meningeal artery embolization during immediate, early, and late follow up: Propensity-score matched cohorts. J Neurointerv Surg. 2023. 15: 943-7
4. Ironside N, Nguyen C, Do Q, Ugiliweneza B, Chen CJ, Sieg EP. Middle meningeal artery embolization for chronic subdural hematoma: A systematic review and meta-analysis. J Neurointerv Surg. 2021. 13: 951-7
5. Izawa D, Matsumoto H, Nishiyama H, Toki N, Kawaguchi T, Yako R. Efficacy of middle meningeal artery embolization for organized chronic subdural hematoma. J Neuroendovasc Ther. 2019. 13: 321-8
6. Kan P, Maragkos GA, Srivatsan A, Srinivasan V, Johnson J, Burkhardt JK. Middle meningeal artery embolization for chronic subdural hematoma: A multi-center experience of 154 consecutive embolizations. Neurosurgery. 2021. 88: 268-77
7. Kanda Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013. 48: 452-8
8. Khorasanizadeh MH, Shutran M, Garcia A, EnriquezMarulanda A, Moore J, Ogilvy CS. Middle meningeal artery embolization for treatment of chronic subdural hematomas: Does selection of embolized branches affect outcomes?. J Neurosurg. 2022. 138: 1494-502
9. Kosaka T, Ikeda N, Furuse M, Nonoguchi N, Hiramatsu R, Yagi R. Refractory chronic subdural hematoma associated with Dural metastasis of lung adenocarcinoma treated with endovascular embolization for the middle meningeal artery: A case report and review of the literature. World Neurosurg. 2020. 133: 256-9
10. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: A series of 60 cases. Neurosurgery. 2019. 85: 801-7
11. Lutz K, Kamenova M, Schaedelin S, Guzman R, Mariani L, Fandino J. Time to and possible risk factors for recurrence after burr-hole drainage of chronic subdural hematoma: A subanalysis of the cSDH-drain randomized controlled trial. World Neurosurg. 2019. 132: e283-9
12. Mowla A, Abdollahifard S, Farrokhi A, Yousefi O, Valibeygi A, Azami P. Middle meningeal artery embolization with liquid embolic agents for chronic subdural hematoma: A systematic review and meta-analysis. J Vasc Interv Radiol. 2023. 34: 1493-500.e7
13. Paro MR, Ollenschleger MD, Fayad MF, Bulsara KR, Stoltz P, Martin JE. Middle meningeal artery embolization for primary treatment of a chronic subdural hematoma in a pediatric patient: A systematic review of the literature and case report. Oper Neurosurg. 2023. 24: 3-10
14. Poon MT, Al-Shahi Salman R. Association between antithrombotic drug use before chronic subdural haematoma and outcome after drainage: A systematic review and meta-analysis. Neurosurg Rev. 2018. 41: 439-45
15. Salem MM, Kuybu O, Hoang AN, Baig AA, Khorasanizadeh M, Baker C. Middle meningeal artery embolization for chronic subdural hematoma: Predictors of clinical and radiographic failure from 636 embolizations. Radiology. 2023. 307: e222045
16. Sattari SA, Yang W, Shahbandi A, Feghali J, Lee RP, Xu R. Middle meningeal artery embolization versus conventional management for patients with chronic subdural hematoma: A systematic review and meta-analysis. Neurosurgery. 2023. 92: 1142-54
17. Stanisic M, Aasen AO, Pripp AH, Lindegaard KF, RammPettersen J, Lyngstadaas SP. Local and systemic pro-inflammatory and anti-inflammatory cytokine patterns in patients with chronic subdural hematoma: A prospective study. Inflamm Res. 2012. 61: 845-52
18. Yang W, Huang J. Chronic subdural hematoma: Epidemiology and natural history. Neurosurg Clin N Am. 2017. 28: 205-10
19. Zhang P, Li Y, Huang J, Zhang H. Chronic subdural haematoma in antithrombotic cohorts: Characteristics, surgical outcomes, and recurrence. Br J Neurosurg. 2020. 34: 408-15