- Department of Neurosurgery, Nara Medical University, Kashihara, Nara, Japan
- Division of Central Radiation, Nara Medical University, Kashihara, Nara, Japan
Correspondence Address:
Ichiro Nakagawa, M.D., Ph.D. Department of Neurosurgery, Nara Medical University, Shijocho, Kashihara, Nara, Japan.
DOI:10.25259/SNI_698_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masashi Kotsugi1, Kengo Konishi2, Shohei Yokoyama1, Ai Okamoto1, Kenta Nakase1, Ryosuke Maeoka1, Ryosuke Matsuda1, Ichiro Nakagawa1. Transarterial embolization for anterior cranial fossa dural arteriovenous fistula based on multi-modal three-dimensional imaging. 25-Oct-2024;15:386
How to cite this URL: Masashi Kotsugi1, Kengo Konishi2, Shohei Yokoyama1, Ai Okamoto1, Kenta Nakase1, Ryosuke Maeoka1, Ryosuke Matsuda1, Ichiro Nakagawa1. Transarterial embolization for anterior cranial fossa dural arteriovenous fistula based on multi-modal three-dimensional imaging. 25-Oct-2024;15:386. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13161
Abstract
Background:Dural arteriovenous fistula (DAVF) in the anterior cranial fossa (ACF) is known to show a high risk of intracranial hemorrhage. Recently, multi-modal fusion imaging with computed tomography angiography, computed tomography venography, and three-dimensional (3D) rotation angiography have been used preoperatively to ensure anatomical safety. We report on endovascular treatment as a first-line approach for ACFDAVF based on the understanding of vascular anatomy obtained from multi-modal fusion imaging.
Methods:All patients with ACF-DAVF treated endovascularly as a first-line approach were included in this study. Analyses took into account complications (particularly visual function), immediate angiographic outcomes, and follow-up findings in consecutive patients with ACF-DAVF treated with interventional treatment based on multi-modal fusion imaging.
Results:Five patients with ACF-DAVF underwent six sessions of transarterial embolization (TAE) in our institution. The five male patients (mean age, 74.5 years; range, 60–84 years) were treated with liquid embolic agents (Onyx, four procedures; n-butyl 2-cyanoacrylate, two procedures). No difference was seen between preoperative image evaluation and image evaluation during the endovascular procedure, and in all cases, a microcatheter was navigated into a target artery assumed from preoperative multi-modal imaging, allowing treatment completion in a single procedure. In all cases, the shunt disappeared completely and visual function after procedure was maintained. At the last follow-up, all patients showed a modified Rankin scale score of 0 or 1 with no recurrences.
Conclusion:Multi-modal fusion imaging facilitates a 3D understanding of the vascular anatomy, allowing TAE as the first-line treatment for ACF-DAVF.
Keywords: Anterior cranial fossa, Endovascular, Multi-modal 3D imaging, Transarterial embolization
INTRODUCTION
Dural arteriovenous fistula (DAVF) located in the anterior cranial fossa (ACF) is rare, representing 3.7–7% of all DAVFs.[
One problem with TAE is that feeders for ACF-DAVF include ethmoidal branches of the ophthalmic arteries (OAs), requiring navigation of the microcatheter to a distal position near the shunt to avoid blindness. Multi-fusion imaging comprising computed tomography angiography (CTA), computed tomography venography (CTV), and three-dimensional (3D) rotation angiography had been used preoperatively in all included cases to ensure anatomical safety. The use of multi-modal imaging enables better visualization of the ACF-DAVF pathophysiology both pre- and intraoperatively, improving treatment safety and certainty. We report on cases treated using endovascular treatment as a first-line approach for ACF-DAVF based on an advanced understanding of the vascular anatomy using multi-modal fusion imaging.
MATERIALS AND METHODS
From 2021 to 2023, all patients with ACF-DAVF treated using endovascular techniques as a first-line approach in our institution were included in this study. This retrospective observational study was based on the criteria of the Strengthening the Reporting of Observational Studies in Epidemiology statement. The Institutional Review Board at our hospital approved this study (approval no. 2580). Analyses took into account complications (particularly those involving visual function), immediate angiographic outcomes, and follow-up findings for consecutive patients with ACF-DAVF treated using interventional treatment.
Radiological imaging condition
Multi-modal 3D fusion imaging with 3D digital subtraction angiography (DSA), magnetic resonance (MR) techniques, and CTA were applied for preoperative evaluation of the angioarchitecture in each patient.
Figure 1:
(a) Maximum intensity projection images are used to follow blood vessels to clarify connections, including feeders for small blood vessels, and to create images. (b) Each feeder should be color-coded to facilitate visualization of the run. Three-dimensional fusion image of anterior cranial fossa-dural arteriovenous fistula for preoperative evaluation utilizing SYNAPSE VINCENT software (Fujifilm Co., Tokyo, Japan).
Endovascular procedures
Neurointerventionists performed all procedures. Unfractionated heparin was administered during the procedure to maintain an activated clotting time of over 300 seconds. The transarterial approach was performed in the standard fashion. An 8-Fr guiding catheter was placed in the appropriate internal carotid artery. A microcatheter was then navigated over the microwire, and selective catheterization of the target artery was performed. A DAC was used to stabilize the microcatheter.
Preoperative multi-fusion imaging was used for catheter navigation to confirm the vascular run and provide a reference for access. These images were also used as a guide to help with wire operation by navigating to the target artery.
Either Onyx or n-butyl 2-cyanoacrylate (NBCA) was used as the embolic agent.
RESULTS
CASE DESCRIPTIONS
Case 1
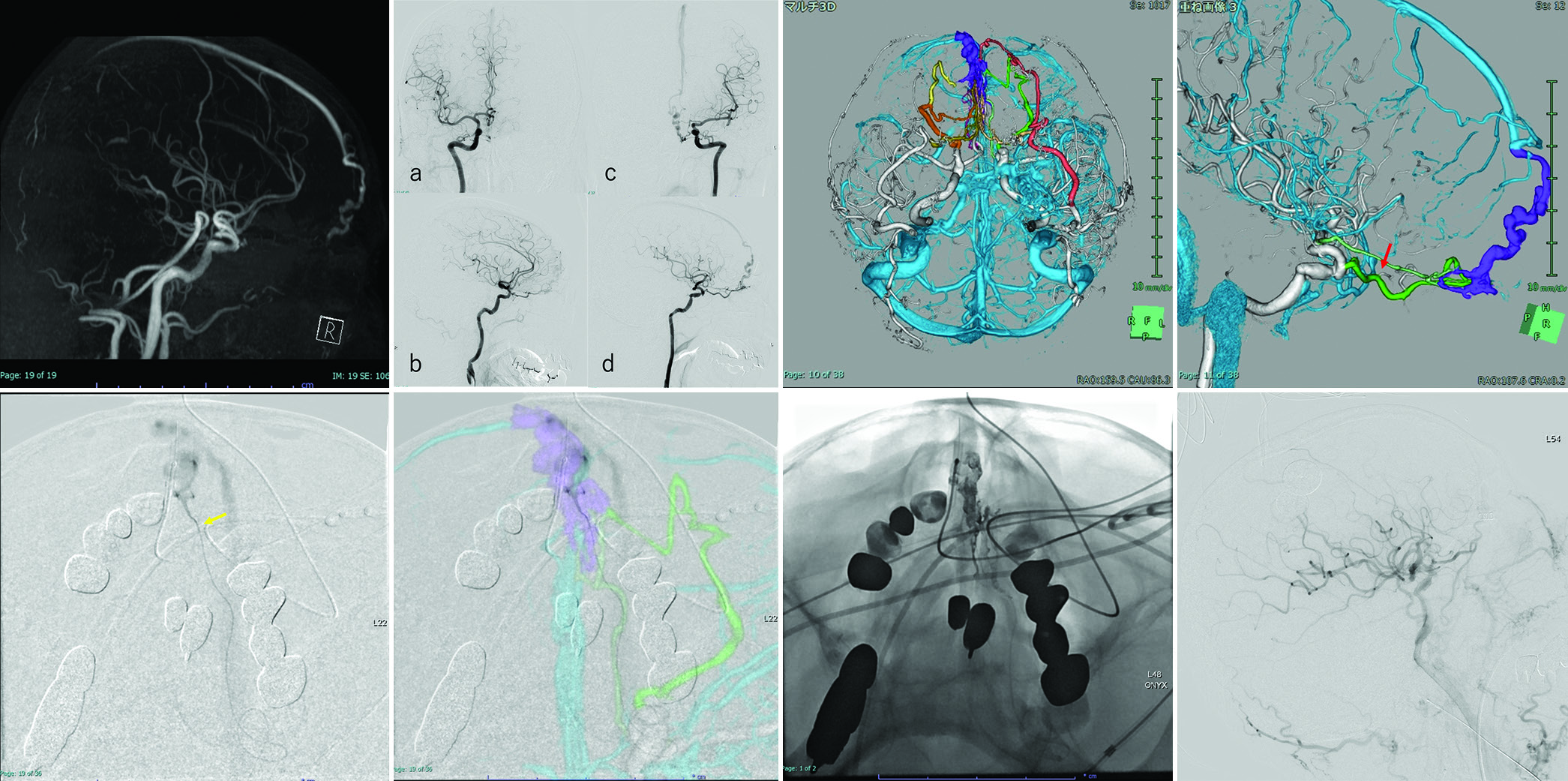
An 84-year-old man presented to the hospital complaining of dizziness. MR imaging of the brain revealed ACF-DAVF [
Figure 2:
(a) Brain magnetic resonance angiography and (b) Digital subtraction angiography reveal anterior cranial fossa-dural arteriovenous fistula. (a and b) Right ICA angiography. (c and d) Left ICA angiography. (c and d) A three-dimensional (3D) reconstructed vascular image visually aids in understanding the 3D angioarchitecture of the shunt. Color coding: Yellow, right anterior ethmoidal artery; orange, right posterior ethmoidal artery (PEA); red, left maxillary artery; green, left PEA; light green, left anterior cerebral artery (ACA). Red arrow: Beginning of the central retinal artery. (e) A front view shows the location of catheters navigated to the shunt through the left ACA. Yellow arrow: the microcatheter close to the shunt. (f) Intraoperative image with superimposed multi-fusion imaging. Image visualization is helped by making the multi-fusion imaging and intraoperative views the same. (g) Onyx penetrates the draining vein beyond the shunt pouch. (h) The shunt point is completely occluded by backflow from the shunt pouch to each feeder, and the shunt disappears.
While reading CTA, CTV, and DSA images, two neurointerventionists and a radiologist collaborated to create multi-modal fusion imaging. The location of the central retinal artery was confirmed based on the resulting images. Multi-modal imaging was reconstructed for preoperative evaluation [
The procedure was performed under general anesthesia. Long 8-Fr sheaths were inserted into the right femoral artery. An 8-Fr guiding catheter (RoadMaster; Goodman, Aichi, Japan) was positioned at the origin of the left ICA. First, with the left 8-Fr Roadmaster, a 3.4-Fr Tactics catheter (Technorat Corporation, Aichi, Japan) was navigated with the aid of a DeFrictor bull microcatheter (Medico’s Hirata, Osaka, Japan) and Tenrou1014 guidewire (Kaneka Medix Corp., Osaka, Japan) close to the bifurcation of the left ACA. Moreover, the DeFrictor bull microcatheter (Medico’s Hirata) and CHIKAI ×10 guidewire (Asahi Intecc, Aichi, Japan) were inserted through the left ACA as close to the shunt point as possible [
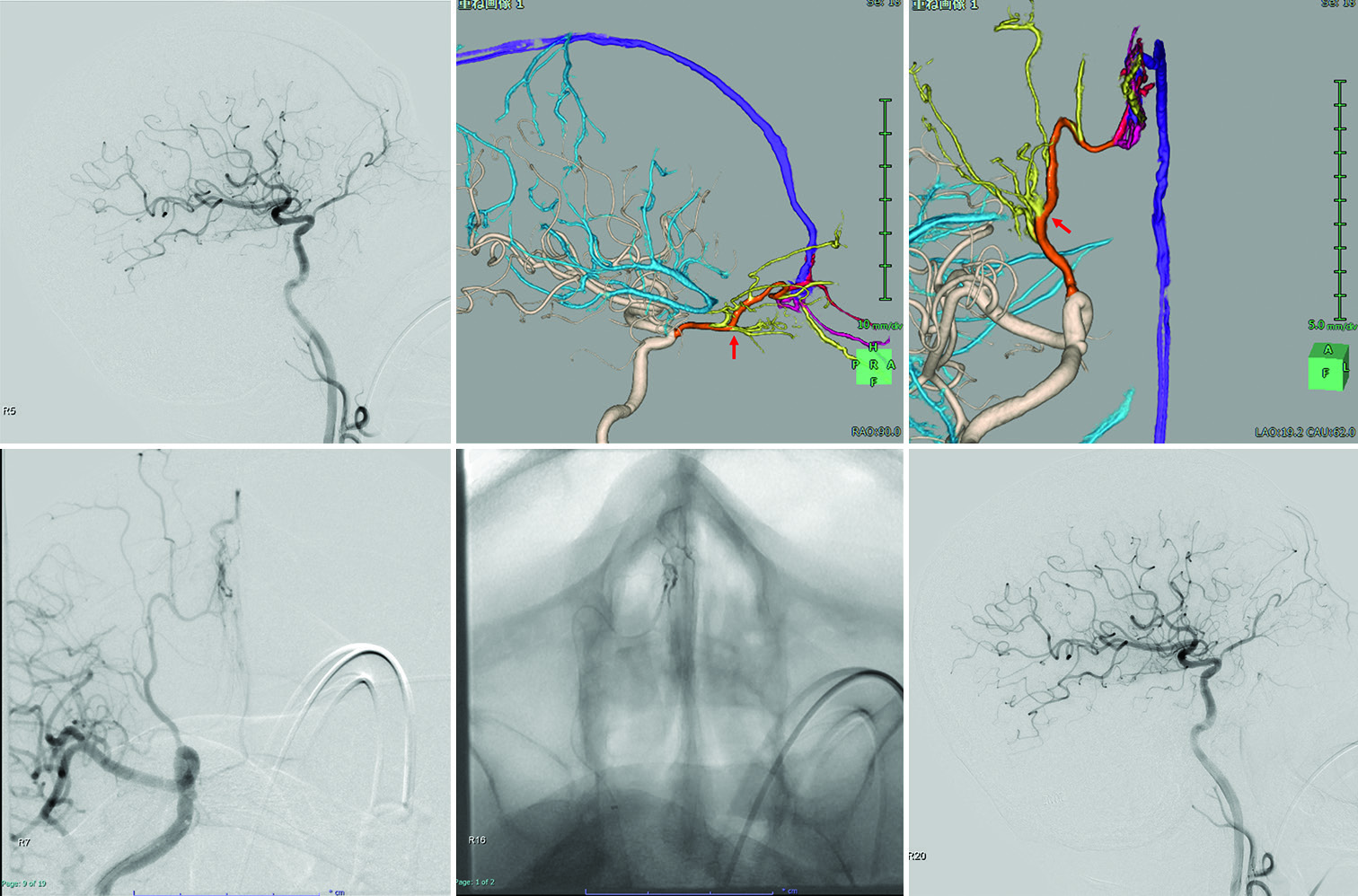
Case 2
A 74-year-old man presented to the hospital complaining of a headache. MR imaging of the brain revealed ACF-DAVF, and he was referred to our department. DSA confirmed an ACFDAVF with a single feeder, arising from the right OA with cortical venous reflux into the SSS through the right anterior fronto-orbital vein (Borden type III, Cognard type IV) [
Figure 3:
(a) Digital subtraction angiography reveals anterior cranial fossa-dural arteriovenous fistula. (b and c) A three-dimensional (3D) reconstructed vascular image visually aids in understanding the 3D angioarchitecture of the shunt. The right posterior ethmoid arteries feed the shunt and drain the superior sagittal sinus through dilated cortical veins. Red arrow (b): Beginning of the central retinal artery. The right posterior ethmoidal artery (c; red arrow) is not tortuous and is close to the shunt point. (d and e) The front view shows the location of catheters navigated to the shunt through the right ophthalmic artery. The microcatheter is close to the shunt. The intermediate distal-access catheter is located in the ophthalmic portion of the right internal carotid artery. (f) The shunt point is completely occluded by backflow from the shunt pouch to each feeder, and the shunt disappears.
The procedure was performed under general anesthesia. A long, 8-Fr sheath was inserted into the right femoral artery. An 8-Fr guiding catheter (FUBUKI; Asahi Intecc) was positioned at the origin of the right ICA. First, using the 8-Fr FUBUKI (Asahi Intecc), a 3.4-Fr Tactics plus catheter (Technorat Corporation, Aichi, Japan) was navigated with the aid of a DeFrictor bull microcatheter (Medico’s Hirata) and Tenrou1014 guidewire (Kaneka Medix Corp.) near to the bifurcation of the right OA. Moreover, a DeFrictor bull microcatheter (Medico’s Hirata) and CHIKAI ×10 guidewire (Asahi Intecc) were inserted through the right PEA as close to the shunt point as possible. Identifying which feeder should be refluxed and where reflux would likely be dangerous was made easy when multi-modal fusion imaging and the intraoperative view were superimposed [
DISCUSSION
No difference was evident between preoperative multi-modal 3D image evaluation and image evaluation during IVR, with all cases involving a microcatheter navigated into a target artery assumed from preoperative multi-modal imaging, allowing treatment to be completed in a single procedure. The preoperative consideration in detail was that using multi-modal 3D imaging, TAE for ACF-DAVF can be performed safely while providing high complete occlusion rates.
Since direct surgery has demonstrated positive clinical outcomes, surgical treatment has been regarded as the major treatment option for ACF-DAVF over the years.[
On the other hand, the safety and efficacy of endovascular treatment for ACF-DAVF have been extensively reported. [
Device improvement is one factor that has enabled the expansion of the indications for TAE. With the development of highly guided DACs and microcatheters, devices can now be directed close to the shunt location. Another reason IVR is feasible is the availability of Onyx, a non-adhesive embolic agent that may be repeatedly injected and paused to overflow into multiple feeders for embolization. Transarterial treatment of DAVFs using non-adhesive liquid embolic agents as the primary embolic agents is well established. Reports of treatment using Onyx for DAVF have recently increased.[
The first preoperative consideration is how to avoid the complication of blindness. Confirming the bifurcation of the central retinal artery on multi-modal 3D imaging is crucial. Multi-modal imaging is performed before transarterial treatment to assess which placements are acceptable and thus avoid regurgitation into the central retinal artery. Although the introduction of Onyx and technological advances have made the disease treatable with IVR, multi-modal fusion imaging is now being used to increase safety and efficacy. Multi-modal 3D images provide visual information to the endovascular surgeon and allow all staff involved in the IVR procedure to see the same image, whereas conventional intraoperative imaging alone is insufficient.
Second, target feeders that can be used to navigate close enough to the shunt point are important. TAE can be highly effective and curative if a catheter can be inserted sufficiently close to the fistulous connection.[
Multi-modal imaging is an advantageous tool for keeping not only the surgeon but also the entire treatment team on the same page. Catheter guidance can be predicted using visual confirmation of the vascular architecture. Although quantifying a safe reflux distance is difficult, multi-modal fusion images offer an indicator.
Some issues with 3D fusion images remain. Because the images are created artificially, some degree of error is possible. Fortunately, no image errors that affected the procedures were encountered in this case series, but arterial and venous images in the shunt can be confused in complex, intricate situations. In the future, preoperative multi-modal 3D images could potentially be linked to actual intraoperative images, which would furnish operators with even better indicators to improve the safety and efficacy of treatment further.
CONCLUSION
Advances in DAC and microcatheters and the expansion of indications for Onyx have made ACF-DAVF reliably curable using TAE. Multi-modal fusion imaging facilitates a 3D understanding of the vascular anatomy, helping to make endovascular treatment the first-line treatment for ACFDAVF.
Ethical approval
The Institutional Review Board approved the research/study at Nara Medical University Hospital, number 2580, dated January 01, 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agid R, Terbrugge K, Rodesch G, Andersson T, Söderman M. Management strategies for anterior cranial fossa (ethmoidal) dural arteriovenous fistulas with an emphasis on endovascular treatment. J Neurosurg. 2009. 110: 79-84
2. Cannizzaro D, Peschillo S, Cenzato M, Pero G, Resta MC, Guidetti G. Endovascular and surgical approaches of ethmoidal dural fistulas: A multicenter experience and a literature review. Neurosurg Rev. 2018. 41: 391-8
3. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 194: 671-80
4. Cognard C, Januel AC, Silva NA, Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: New management using Onyx. AJNR Am J Neuroradiol. 2008. 29: 235-41
5. Dabus G, Kan P, Diaz C, Pabon B, Andres-Mejia J, Linfante I. Endovascular treatment of anterior cranial fossa dural arteriovenous fistula: A multicenter series. Neuroradiology. 2021. 63: 259-66
6. Halbach VV, Higashida RT, Hieshima GB, Wilson CB, Barnwell SL, Dowd CF. Dural arteriovenous fistulas supplied by ethmoidal arteries. Neurosurgery. 1990. 26: 816-23
7. Hiramatsu M, Sugiu K, Hishikawa T, Nishihiro S, Kidani N, Takahashi Y. Results of 1940 embolizations for dural arteriovenous fistulas: Japanese Registry of Neuroendovascular Therapy (JR-NET3). J Neurosurg. 2019. 133: 166-73
8. Hou K, Ji T, Guo Y, Xu B, Xu K, Yu J. Current status of endovascular treatment for dural arteriovenous fistulas in the superior sagittal sinus region: A systematic review of the literature. World Neurosurg. 2019. 122: 133-43
9. Inoue A, Tagawa M, Kumon Y, Watanabe H, Shoda D, Sugiu K. Ethmoidal dural arteriovenous fistula with unusual drainage route treated by transarterial embolization. J Neurointerv Surg. 2015. 7: e15
10. Jee TK, Lee YW, Yeon JY, Kim KH, Jeon P, Kim JS. Surgical strategy for ethmoidal dural arteriovenous fistula. World Neurosurg. 2022. 164: e91-8
11. Kakarla UK, Deshmukh VR, Zabramski JM, Albuquerque FC, McDougall CG, Spetzler RF. Surgical treatment of high-risk intracranial dural arteriovenous fistulae: Clinical outcomes and avoidance of complications. Neurosurgery. 2007. 61: 447-57
12. Lin N, Brouillard AM, Mokin M, Natarajan SK, Snyder KV, Levy EI. Direct access to the middle meningeal artery for embolization of complex dural arteriovenous fistula: A hybrid treatment approach. J Neurointerv Surg. 2015. 7: e24
13. Lv X, Li Y, Liu A, Lv M, Jiang C, Wu Z. Endovascular embolization of dural arteriovenous fistulas of the anterior cranial fossa: Three case reports. Neurol Res. 2008. 30: 852-9
14. Meneghelli P, Pasqualin A, Lanterna LA, Bernucci C, Spinelli R, Dorelli G. Surgical treatment of anterior cranial fossa dural arterio-venous fistulas (DAVFs): A twocentre experience. Acta Neurochir (Wien). 2017. 159: 823-30
15. Robert T, Blanc R, Smajda S, Ciccio G, Redjem H, Bartolini B. Endovascular treatment of cribriform plate dural arteriovenous fistulas: Technical difficulties and complications avoidance. J Neurointerv Surg. 2016. 8: 954-8
16. Spiotta AM, Hawk H, Kellogg RT, Turner RD, Chaudry MI, Turk AS. Transfemoral venous approach for Onyx embolization of anterior fossa dural arteriovenous fistulae. J Neurointerv Surg. 2014. 6: 195-9
17. Zhang L, Wang H, Pan Y, Mao L, Ding K, Zhu J. Clinical characteristics and microsurgery treatment of anterior cranial fossa dural arteriovenous fistula. J Craniofac Surg. 2019. 30: e701-3