- Department of Interventional Neuroradiology, Hospital Beneficência Portuguesa de São Paulo, São Paulo, Brazil
- Department of Neuroscience, Miami Neuroscience Institute Baptist Hospital of Miami, Miami, United States
- Department of Interventional Neuroradiology Service, Hospital das Clínicas da Faculdade de Medicina da USP, São Paulo, Brazil
Correspondence Address:
Rafael Trindade Tatit, Department of Interventional Neuroradiology, Hospital Beneficência Portuguesa de São Paulo, São Paulo, Brazil.
DOI:10.25259/SNI_442_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rafael Trindade Tatit1, Guilherme Dabus2, Thomas Alexandre Yasuda3, Carlos Eduardo Baccin1. Transarterial embolization of petrosal dural arteriovenous fistula (dAVF): Feasible and successful in the post-Onyx era. 01-Nov-2024;15:395
How to cite this URL: Rafael Trindade Tatit1, Guilherme Dabus2, Thomas Alexandre Yasuda3, Carlos Eduardo Baccin1. Transarterial embolization of petrosal dural arteriovenous fistula (dAVF): Feasible and successful in the post-Onyx era. 01-Nov-2024;15:395. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13197
Abstract
Background: Intracranial dural arteriovenous fistula (dAVF) is a rare arteriovenous malformation with potentially severe complications. This study investigates the efficacy and safety of transarterial embolization (TAE) in treating petrous dAVFs through a retrospective analysis and literature review.
Case Description: A retrospective analysis of six patients with petrous dAVFs treated with TAE was conducted, accompanied by a systematic literature review to evaluate treatment outcomes. Data collection included patient characteristics, clinical presentation, Borden–Shucart and Cognard classifications, treatment specifics, and overall outcomes. TAE, particularly utilizing Onyx, demonstrated favorable outcomes in our six patients. Regarding literature review results, 102 articles were identified through PubMed Mesh tool search, but only five were included after careful evaluation. In addition, one article was manually added after searching for the remaining articles. Combining our six cases with literature findings, 79.8% (n = 75) of patients undergoing TAE achieved a cure with the technique, though Onyx was reported in only 13.9% (n = 13) of TAE cases. Complications were observed in 11.7% (n = 11) of TAE cases.
Conclusion: Our presented cases and literature review suggest that the TAE of dAVFs is feasible and curative for selected cases of petrous dAVFs. However, the complexity of these lesions and the availability of other treatment modalities should be taken into consideration to optimize cure rates and patient outcomes.
Keywords: Arteriovenous malformation, Dural arteriovenous fistula, Facial nerve ischemia, Onyx embolization, Petrous dural arteriovenous fistula (dAVF), Transarterial embolization, Vascular malformation
INTRODUCTION
Background
Intracranial dural arteriovenous fistula (dAVF) represents a subtype of arteriovenous malformations (AVMs) where dural arteries directly connect into veins within the walls of dural venous sinuses, bypassing capillary beds. The incidence is relatively low, approximately 0.15–0.29/100,000 people annually,[
Most risk stratification schemes rely on the angiographic characteristics of dAVFs, specifically considering the presence or absence of retrograde cortical venous drainage (CVD).[
The primary goal in dAVF treatment is complete disconnection from venous drainage, achievable through endovascular, surgical, or stereotactic radiosurgery methods. Although endovascular treatment has become the first-line approach for most patients,[
Objectives
This investigation seeks to present a retrospective analysis of treated cases of petrous dAVF using transarterial embolization (TAE), shedding light on the effectiveness and safety of this approach. Furthermore, a literature review was conducted on PubMed to evaluate previously published cases of dAVF treated with TAE.
METHODS
Study design
Retrospective analysis of cases of petrous dAVF treated with TAE conducted at two specialized centers, from January 2020 to November 2023. Six patients with at least one follow-up with cerebral angiography within 3 months of the procedure were identified. In addition, a literature review was conducted on PubMed to evaluate previously published cases of petrous dAVF treated with TAE. Only studies describing a minimum of three cases using this technique were included. Given the inclusion of a small number of cases and the utilization of previously collected and anonymized data obtained with the requisite patient consent, ethical committee approval was deemed unnecessary.
Variables and outcomes
Patient data encompassing age, gender, clinical presentation, Borden–Shucart type, Cognard type, arterial feeders, venous drainage, details of the first treatment, subsequent treatments, curative treatment, initial angiographic outcome, final angiographic outcome, and treatment complications were extracted from medical records and neuroimaging reviews. In addition, magnetic resonance angiography reports were scrutinized for comprehensive final follow-up data. The study focused on these variables to provide a succinct yet comprehensive analysis of key factors and outcomes pertinent to the research objectives.
Statistical analysis
We present the variables, all of which are categorical, in the form of proportions or percentages.
RESULTS
Baseline patient characteristics
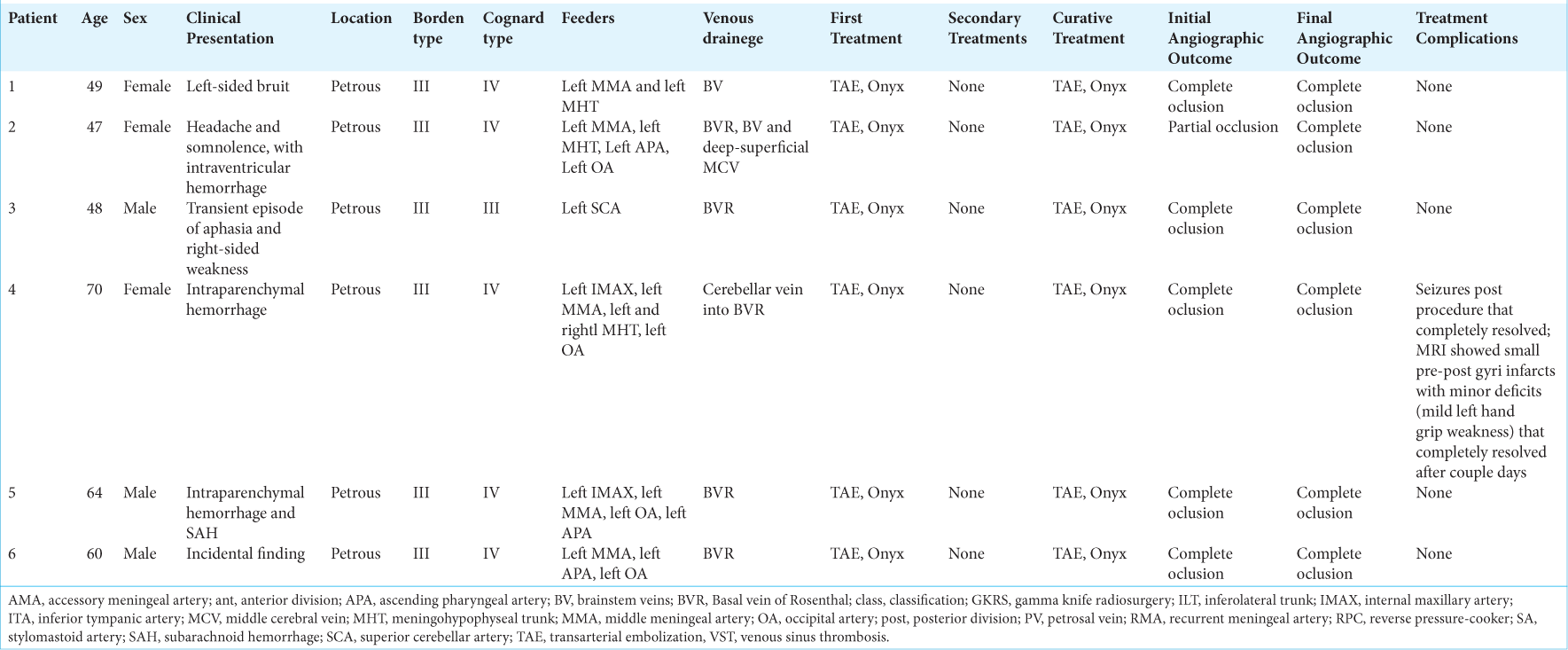
A total of 6 patients with petrous dAVF treated with TAE were analyzed [
Figure 1:
(a) Selective left external carotid artery angiogram showing a petrosal dural arteriovenous fistula (dAVF) (arrow) fed by branches of the left middle meningeal artery draining into superior petrosal vein, the basal vein of Rosenthal and deep Sylvian vein. (b) Plain X-ray showing the Onyx cast after embolization (arrow). (c) Left common carotid artery 10-month follow-up angiography showing complete occlusion of the petrosal dAVF.
Figure 2:
(a) Head computed tomography angiography showing intraventricular hemorrhage (arrowhead) with venous aneurysms and ectasia of brainstem veins (arrow) suggestive of a dural arteriovenous fistula (dAVF). (b) Selective left external carotid artery angiography and (c) 3D MIP reconstructions showing a left petrosal dAVF supplied by branches of the left middle meningeal artery draining into the brainstem and temporal veins with venous aneurysms (arrows). (d) Selective left internal carotid artery (ICA) angiogram without subtraction showing the Onyx cast adjacent to the petrosal bone and branches from the ICA previously supplying the arteriovenous shunt (arrow). (e) Common carotid artery final angiogram early arterial and (f) early venous phases showing complete occlusion of the dural arteriovenous shunt and stasis of dilated branches of the meningohypophyseal trunk (arrow) previously supplying the fistula.
Baseline dAVF characteristics
All dAVFs analyzed in this study were located in the petrous region, encompassing superior petrosal sinus dAVFs, inferior petrosal sinus dAVFs, and petrous apex dAVFs. According to the Borden–Shucart classification, all cases were classified as type III. Among them, one was categorized as Cognard type III (17%), and the remaining five were identified as Cognard type IV (83%) [
Regarding vascular supply, only one dAVF was exclusively supplied by one artery, the superior cerebellar artery, while all others received contributions from at least two arteries. The middle meningeal artery supplied five dAVFs (83%), the occipital artery supplied four (67%), the meningohypophyseal trunk supplied three (50%), the ascending pharyngeal artery supplied three (50%), and the internal maxillary artery supplied two (33%).
In terms of venous drainage, the basal vein of Rosenthal was the main drainage in five dAVFs (83%). The brainstem veins drained two cases (33%), and the deep-superficial middle cerebral veins were involved in one case (17%).
Procedure characteristics and outcomes
All cases presented in this study were treated with TAE using Onyx as the primary and only therapeutic technique. In one case (17%), the initial post-procedural angiographic control demonstrated partial occlusion of the dAVF, while all five remaining cases (83%) exhibited complete occlusion in the initial angiographic control. One patient experienced post-procedural complications, including seizures that were completely resolved. Magnetic resonance imaging revealed small pre and post-central gyri infarcts with minor deficits characterized by mild left-hand grip weakness that completely resolved after a couple of days. In the latest angiographic follow-up, all patients showed complete occlusion of the dAVF.
Literature review
A total of 102 articles were identified through the PubMed Mesh tool search, from which five articles were selected after full-text review.[
Combining the results from these articles with our six presented cases [
The initial treatment was TAE in 80.2% (n = 93), surgery in 15.7% (n = 17), transvenous embolization (TVE) in 4.3% (n = 5), Gamma Knife radiosurgery (GK) in 0.9% (n = 1), and 0.9% (n = 1) information regarding the first treatment was unavailable. Regarding subsequent treatments, only 0.9% (n = 1) underwent TAE, 13.8% (n = 16) had surgery, and 2.6% (n = 3) underwent TVE.
Regarding the curative treatment, 79.8% (n = 75) of patients undergoing TAE achieved cure with the technique, 100% (n = 33) with surgery, 50% (n = 3) with TVE, and 100% (n = 1) with GK. Onyx was used in only 13.9% (n = 13) of the cases undergoing TAE. Complications were observed in 11.7% (n = 11) of the cases undergoing TAE, 9.1% (n = 3) of surgery cases, and 20% (n = 2) of cases treated with other techniques. The average follow-up duration was 37.6 months.
DISCUSSION
Efficacy and Strategies of TAE for Petrous dAVFs
TAE strategies for intracranial dAVFs are designed to achieve closure at both the fistulous point and the proximal aspect of the draining vein. However, several authors have questioned the effectiveness of TAE as a standalone curative therapy.[
Before committing to TAE, the feasibility of attaining a suitable distal position with the microcatheter must be assessed. A distal position increases the chance of cure and allows for the preservation of the vasa nervorum proximal to cranial nerves while avoiding external carotid to internal carotid artery anastomoses, ensuring a reasonable safety margin for ethylenevinyl alcohol copolymer arterial reflux.[
Clinical outcomes and advances
An intriguing study by Bhatia et al.[
Limitations and generalizability
Although our review compiled a total of 116 cases of petrous dAVF from six different articles, including our cases, the studies exhibited significant variations that preclude the generalization of the results. Factors such as publication date and the consequent availability of techniques at the time, the materials used (notably, only 13.9% of TAE cases reported the use of Onyx), and, except for Bhatia et al.[
Moreover, there is a substantial risk of selection and publication bias inherent in this study modality, as successfully treated cases with a particular approach are more likely to be published and selectively exposed to the scientific community. Another potential source of systematic error in our review, driven by the selection criteria favoring studies describing at least 3 TAE cases, is the limited inclusion of petrous dAVFs primarily treated with surgery (15.7%). This poses the risk of selectively including cases where surgery was utilized for refractory cases of TAE, potentially biasing the results in favor of surgical treatment.
CONCLUSION
Our findings suggest that TAE using Onyx is a feasible and effective treatment for petrous dAVFs. However, the potential advantages of surgical approaches, as highlighted by existing literature, should not be overlooked. Future studies should aim to compare these treatment modalities directly, taking into account patient-specific factors and anatomical complexities to optimize outcomes. Detailed descriptions of arterial supply and venous drainage are crucial for enhancing treatment planning and reducing complications.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ayad M, Eskioglu E, Mericle RA. Onyx: A unique neuroembolic agent. Expert Rev Med Devices. 2006. 3: 705-15
2. Baharvahdat H, Ooi YC, Kim WJ, Mowla A, Coon AL, Colby GP. Updates in the management of cranial dural arteriovenous fistula. Stroke Vasc Neurol. 2020. 5: 50-8
3. Barnwell SL, Halbach VV, Dowd CF, Higashida RT, Hieshima GB. Dural arteriovenous fistulas involving the inferior petrosal sinus: Angiographic findings in six patients. AJNR Am J Neuroradiol. 1990. 11: 511-6
4. Berenstein A, Lasjaunias P, Ter Brugge KG. Surgical neuroangiography: Vol 2, Clinical and endovascular treatment aspects in adults. Berlin, Heidelberg: Springer; 2004. p.
5. Bhatia KD, Kortman H, Lee H, Waelchli T, Radovanovic I, Schaafsma JD. Facial nerve arterial arcade supply in dural arteriovenous fistulas: Anatomy and treatment strategies. AJNR Am J Neuroradiol. 2020. 41: 687-92
6. Bhatia KD, Lee H, Kortman H, Klostranec J, Guest W, Wälchli T. Endovascular management of intracranial dural arteriovenous fistulas: Transarterial approach. AJNR Am J Neuroradiol. 2022. 43: 324-31
7. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995. 82: 166-79
8. Brown RD, Wiebers DO, Torner JC, O’Fallon WM. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology. 1996. 46: 949-52
9. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 194: 671-80
10. Dawson RC, Joseph GJ, Owens DS, Barrow DL. Transvenous embolization as the primary therapy for arteriovenous fistulas of the lateral and sigmoid sinuses. AJNR Am J Neuroradiol. 1998. 19: 571-6
11. Gandhi D, Chen J, Pearl M, Huang J, Gemmete JJ, Kathuria S. Intracranial dural arteriovenous fistulas: Classification, imaging findings, and treatment. AJNR Am J Neuroradiol. 2012. 33: 1007-13
12. Geibprasert S, Pongpech S, Armstrong D, Krings T. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: Vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol. 2009. 30: 1459-68
13. Gross BA, Albuquerque FC, Moon K, McDougall CG. Evolution of treatment and a detailed analysis of occlusion, recurrence, and clinical outcomes in an endovascular library of 260 dural arteriovenous fistulas. J Neurosurg. 2017. 126: 1884-93
14. Grossberg JA, Cawley CM, editors. Treatment of other intracranial dural arteriovenous fistulas. Youman’s neurological surgery. Netherlands: Elsevier; 2016. p. 3530-6
15. Halbach VV, Higashida RT, Hieshima GB, Mehringer CM, Hardin CW. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol. 1989. 10: 385-92
16. Hwang G, Kang HS, Oh CW, Kwon OK. Surgical obliteration in superior petrosal sinus dural arteriovenous fistula. J Korean Neurosurg Soc. 2011. 49: 222-5
17. Lapresle J, Lasjaunias P. Cranial nerve ischaemic arterial syndromes. A review. Brain. 1986. 109: 207-16
18. Li J, Ren J, Du S, Ling F, Li G, Zhang H. Dural arteriovenous fistulas at the petrous apex. World Neurosurg. 2018. 119: e968-76
19. Liu JK, Dogan A, Ellegala DB, Carlson J, Nesbit GM, Barnwell SL. The role of surgery for high-grade intracranial dural arteriovenous fistulas: Importance of obliteration of venous outflow. J Neurosurg. 2009. 110: 913-20
20. Lv X, Jiang C, Li Y, Wu Z. Results and complications of transarterial embolization of intracranial dural arteriovenous fistulas using Onyx-18. J Neurosurg. 2008. 109: 1083-90
21. Merland JJ, Moret J, Lasjaunias P, Théron J. Cranial osseous and meningeal blood supply (author’s transl). J Neuroradiol. 1977. 4: 335-6
22. Mitsuhashi Y, Aurboonyawat T, Pereira VM, Geibprasert S, Toulgoat F, Ozanne A. Dural arteriovenous fistulas draining into the petrosal vein or bridging vein of the medulla: Possible homologs of spinal dural arteriovenous fistulas. J Neurosurg. 2009. 111: 889-99
23. Ozanne A, Pereira V, Krings T, Toulgoat F, Lasjaunias P. Arterial vascularization of the cranial nerves. Neuroimaging Clin N Am. 2008. 18: 431-9
24. Pabaney AH, Robin AM, Basheer A, Malik G. Surgical management of dural arteriovenous fistula after craniotomy: Case report and review of literature. World Neurosurg. 2016. 89: 731.e7-11
25. Rabinov JD, Yoo AJ, Ogilvy CS, Carter BS, Hirsch JA. ONYX versus n-BCA for embolization of cranial dural arteriovenous fistulas. J Neurointerv Surg. 2013. 5: 306-10
26. Reynolds MR, Lanzino G, Zipfel GJ. Intracranial dural arteriovenous fistulae. Stroke. 2017. 48: 1424-31
27. Satomi J, Satoh K. Epidemiology and etiology of dural arteriovenous fistula. Brain Nerve. 2008. 60: 883-6
28. Westermaier T, Bendszus M, Solymosi L, Roosen K, Ernestus RI. Surgical treatment of dural arteriovenous fistulas of the petrous apex. World Neurosurg. 2012. 77: 591.e7-13
29. Wilson T, Wolfe S, Cohen-Gadol A, editors. Principles of dural arteriovenous fistula surgery. Neurosurgical atlas. Indiana: Neurosurgical Atlas. Inc.; 2016. p.
30. Youssef PP, Schuette AJ, Cawley CM, Barrow DL. Advances in surgical approaches to dural fistulas. Neurosurgery. 2014. 74: S32-41