- Department of Medical Imaging, Fu Jen Catholic University Hospital, New Taipei City, Taipei, Taiwan
- Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan
Correspondence Address:
Hon-Man Liu, Department of Medical Imaging, Fu Jen Catholic University Hospital, New Taipei City, Taiwan.
DOI:10.25259/SNI_133_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hon-Man Liu1,2, Chung-Wei Lee2, Yen-Heng Lin2. Treatment of 23 spinal perimedullary arteriovenous fistulas in a single center: A simple and practical treatment strategy. 23-May-2025;16:196
How to cite this URL: Hon-Man Liu1,2, Chung-Wei Lee2, Yen-Heng Lin2. Treatment of 23 spinal perimedullary arteriovenous fistulas in a single center: A simple and practical treatment strategy. 23-May-2025;16:196. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13573
Abstract
Background: The aim of the study is to present our strategy for stratifying patients with spinal perimedullary arteriovenous fistulas (PMAVFs) and apply the appropriate treatment.
Methods: This retrospective study included 23 patients with PMAVF. We divided the patients into three groups according to the location of the fistula and size of the predominant feeder: Group 1 (dorsal PMAVF, n = 4), Group 2 (nondorsal PMAVF having a predominant feeder through which the smallest coil-deploying microcatheter could pass, n = 6), and Group 3 (nondorsal PMAVF having no feeder through which the smallest available microcatheter could pass, n = 13). Group 1 underwent surgical treatment. All patients in Groups 2 and 3 underwent endovascular treatment with a liquid embolic agent, except one in Group 3, who opted for surgical treatment. Coil was used as a supplementary tool for treating lesions in Group 2. Patients’ basic and clinical characteristics, treatment, and outcome data were recorded.
Results: Six patients were aged n = 11), conus region (n = 7), and cervical spine (n = 5). Of the 18 PMAVFs who underwent endovascular treatment, 100% occlusion was observed in 14, 90% in 3, and 75% in 1. Nineteen patients had complete or partial recovery of neurological deficits. Six patients experienced temporary worsening immediately after treatment but recovered within 3 months. No bleeding or rebleeding was noted after either treatment.
Conclusion: Our simple strategy for stratifying PMAVF for treatment is easy to apply in clinical practice and results in favorable outcomes.
Keywords: Endovascular treatment, Outcome, Patient selection, Perimedullary fistula, Spinal vascular malformation
INTRODUCTION
A spinal perimedullary arteriovenous fistula (PMAVF), or Type IV spinal arteriovenous malformation (AVM), is an uncommon vascular malformation. PMAVF, a direct intradural and extramedullary arteriovenous fistula (AVF), is typically supplied by the anterior spinal artery, posterolateral artery, radiculomedullary artery, or the artery to the terminal filum. Its drainage is accomplished through the superficial perimedullary veins or pial venous network. Approximately 20–31% of spinal vascular malformations are PMAVFs. Spinal medullary AVMs and PMAVFs have similar clinical presentations.[
Previous studies have arbitrarily classified PMAVFs according to the size and number of feeders, fistula size, and morphology of draining veins.[
Locating and eradicating the PMAVF shunt can be highly challenging in microsurgical treatment because PMAVFs are often located between congested veins, thus obscuring the fistula.[
MATERIALS AND METHODS
This retrospective study examined prospectively collected data and used the neuroradiology database to identify patients with a diagnosis of PMAVF. The ethics committee of National Taiwan University Hospital approved this study. Patients’ basic and clinical characteristics, treatment, and outcome data were recorded.
All patients with PMAVF underwent magnetic resonance imaging examination and catheter angiography. The patients with PMAVF were stratified according to the location of PMAVF. We divided the patients into three groups [
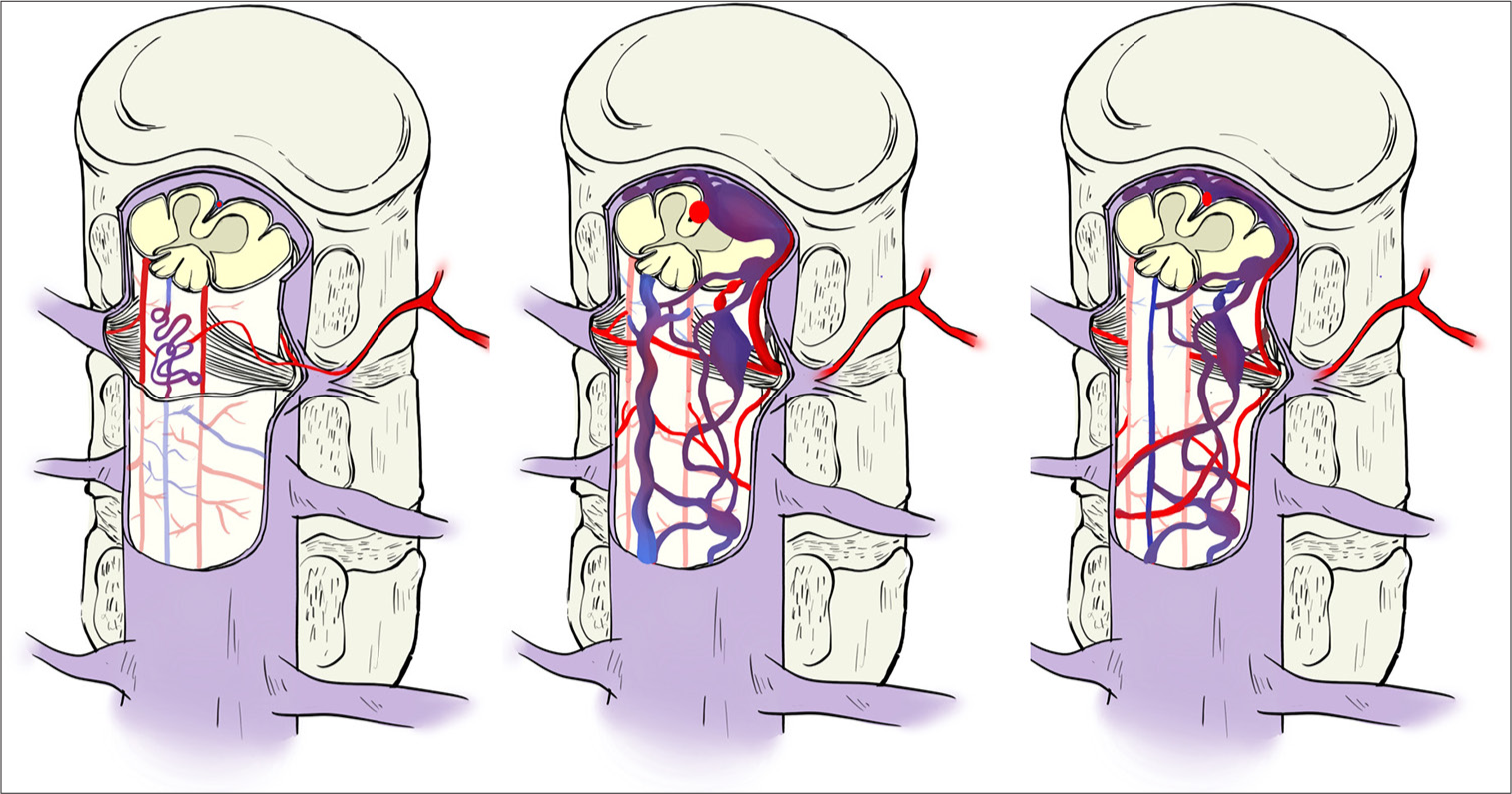
Figure 1:
An illustration contrasting the different groups of perimedullary arteriovenous fistulas (PAMVF). Group 1 (left) is a PAMVF that occurs solely dorsal to the spinal cord. Group 2 (central) is a nondorsal PMAVF having a predominant feeder through which the smallest coil-deploying microcatheter could pass. Group 3 (right) is a nondorsal PMAVF having no feeder through which the smallest available microcatheter could pass.
All patients in Group 1 underwent surgical treatment. All patients in Groups 2 and 3 underwent endovascular treatment, except one patient who opted for microsurgical treatment.
In Group 2, we administered an intravenous bolus of hydrocortisone (5.3 mg/kg) before embolization. This was repeated hourly for 3–7 days.[
Postembolization angiography results were classified as follows: complete occlusion, nearly complete occlusion (only slow residual arteriovenous shunting on angiography), and partial occlusion (with obvious residual arteriovenous shunting on angiography).
Patient outcomes were classified as excellent (complete recovery), improved (partial recovery of neurological signs), unchanged (same neurological status as before treatment), or worse (development of any new neurological signs) at clinical follow-up conducted at least 2 years after treatment.
RESULTS
We identified 156 patients diagnosed as having a spinal vascular malformation, of whom 23 (15 male and 8 female patients) had catheter angiography-confirmed PMAVF. Among them, 3 patients were 1–3 years old, 3 were 3–14 years old, 14 were 15–40 years old, and 3 were 40–56 years old. All patients had muscle weakness (sudden in 9; progressive in 14) as their primary symptom. Of these patients, nine had additional sensations or pain symptoms, and four had urine incontinence. The fistulas were located in the thoracic region (n = 11), conus region (n = 7), and cervical spine (n = 5). Of the included patients, 4 patients had Group 1 lesions, 6 patients had Group 2 lesions, and 13 patients had Group 3 lesions. In five lesions in Group 2, the main feeder of the PMAVFs was either the anterior spinal artery or the artery of Adamkiewicz. All lesions in Group 2 were associated with a dilated venous aneurysm near the fistula site.
Three of the four patients in Group 1 had a single feeding artery. All patients in Group 1 underwent surgery; the outcomes were excellent in two patients, improved in one, and unchanged in one. One patient had spontaneous closure of the fistula during the operation, which was confirmed through postoperative angiography [
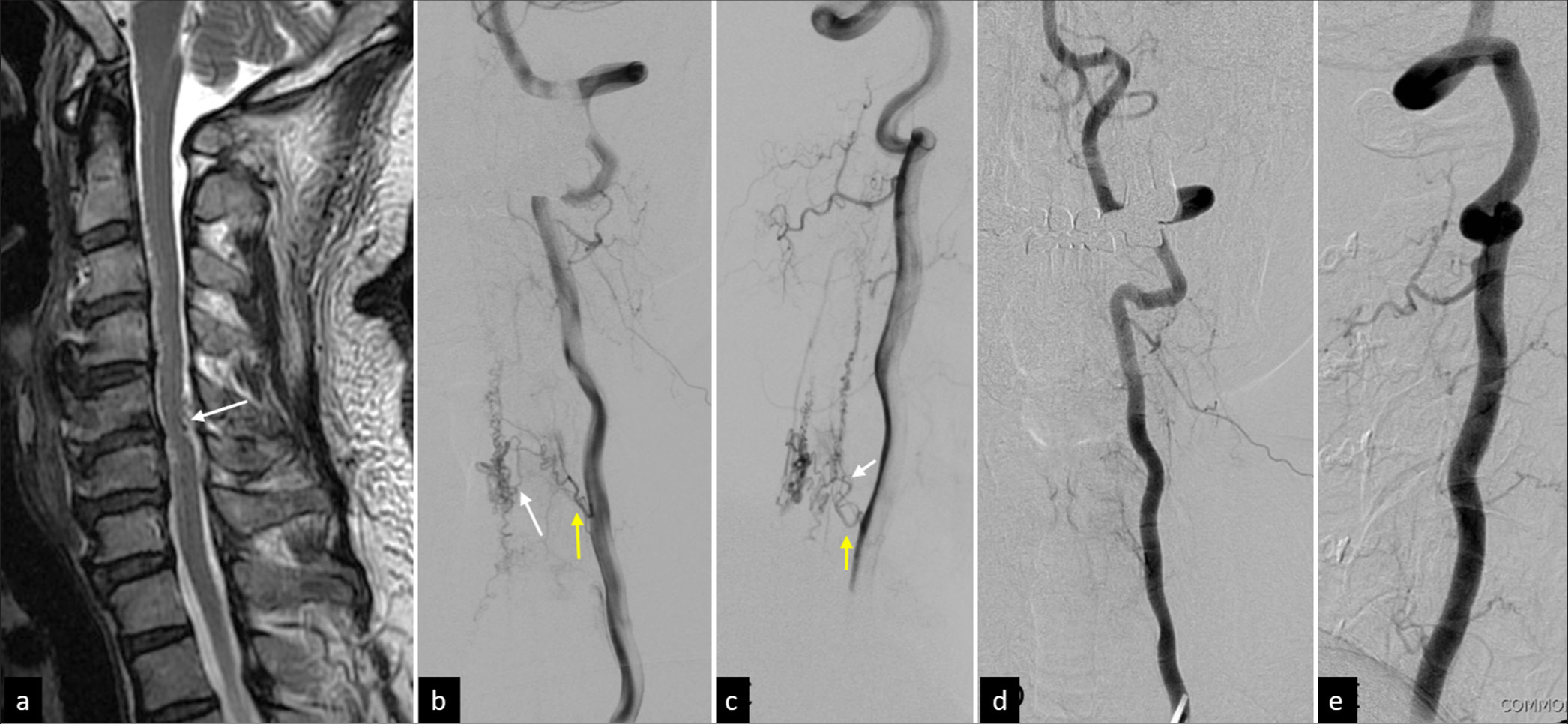
Figure 3:
A 45-year-old man had mild bilateral lower leg weakness and upper extremity numbness for months. Sagittal T2-weighted magnetic resonance imaging revealed (a) an abnormal flow void (arrow) on the dorsal aspect of the C5/6 cord associated with some fine void spots in the ventral aspect of C3/4. (b and c) Lateral view of left vertebral angiography showing a Group 1 perimedullary arteriovenous fistula predominantly supplied by the left C5 radiculomedullary artery (yellow arrow) and posterior spinal artery (white arrow). (d and e) During the surgical treatment of the lesion, the abnormal vasculature and fistula spontaneously disappeared. The follow-up angiography confirmed it. The patient had no more neurological signs after the operation and has remained symptom-free for 6 years.
A liquid embolic agent was used in 18 patients in Groups 2 and 3 (NBCA in 12, Onyx in 3, and both in 2). Two patients received both NBCA and Onyx because of failed occlusion of the proximal drainage veins when using NBCA, and Onyx was used as salvage. Coiling was used in Group 2 [
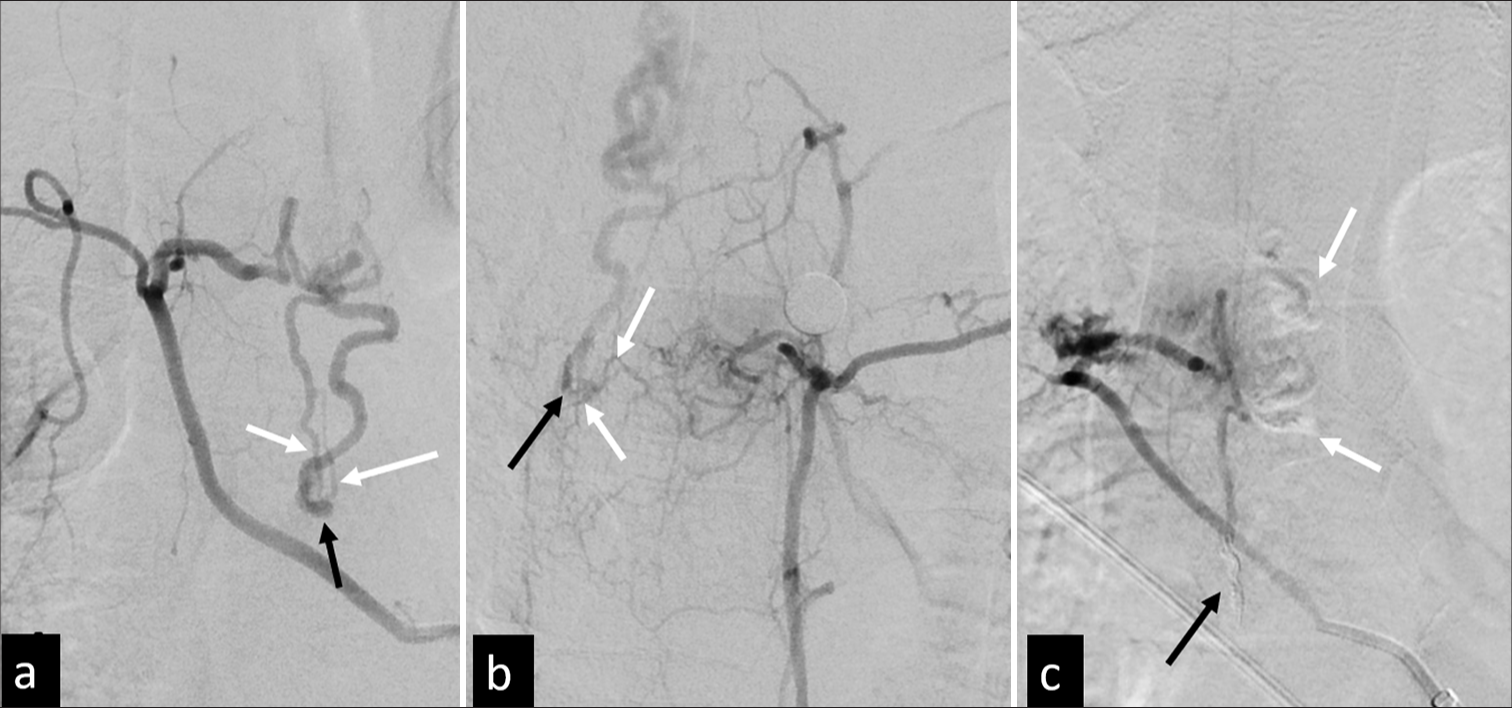
Figure 4:
(a) A 4-year-old boy with left hemiplegia was diagnosed as having a Group 2 perimedullary arteriovenous fistula at the C4-5 level by magnetic resonance (MR). (b and c) On bilateral vertebral angiograms (frontal view b; right, c; left), the main feeding arteries are the bilateral anterior spinal artery (black arrows), with multiple small feeders from segmental radiculomedullary arteries (white arrows). A large venous aneurysm is noted near the fistula site (arrowheads). (d) Through the left anterior spinal artery approach, the microcatheter (black arrows) is navigated into the venous aneurysm, multiple detachable coils are deployed, followed by an Onyx injection, and (e and f) the shunting is completely obliterated. (g and h) Six-month follow-up angiography indicates no evidence of local residual or recurrent disease and (i) is confirmed by 2-year follow-up MR. Coil mass (arrows) is noted without abnormal void signal. The patient became symptom-free at the 3-year follow-up.
Figure 5:
(a and b) A 34-year-old man presented with sudden-onset hemiparesis and loss of sensation in the lower extremities. The clinical diagnosis was Brown–Sequard syndrome below T6. The left T8 intercostal artery angiography reveals a group 2 perimedullary arteriovenous fistula with multiple radiculomedullary feeders (a and b; white arrows) sharing a common drainage (black arrows). (c) The lesion is treated with Onyx (white arrows) with complementary coiling (black arrow) at the distal main feeder. The patient experienced complete recovery and remained symptom-free at the 7-year follow-up.
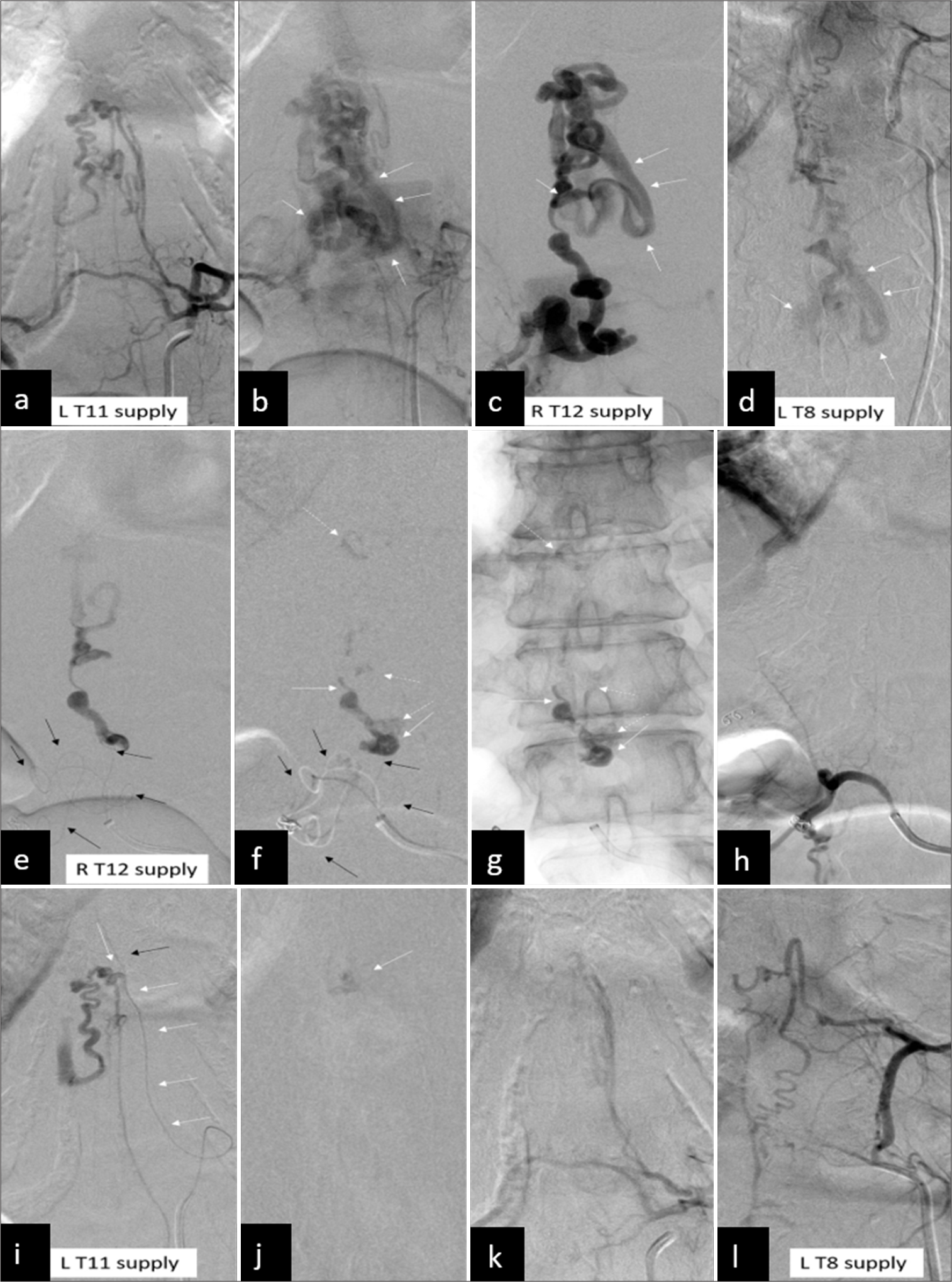
Figure 6:
(a and b) A 37-year-old woman suffered from right lower leg numbness for 1 year. The symptoms then gradually progressed to bilateral lower limb associated with weakness and dysuria. A group 3 perimedullary arteriovenous fistula with multiple feeders from left T11, (c) right T12, and (d) left T8 intercostal arteries. A large common venous drainage (b-d; white arrows) is noted. (e and f) The 1st session of embolization is conducted at the right T12 intercostal artery with a microcatheter (black arrows) advancing to the fistula site as close as possible. (f and g) The feeder (f and g, white arrows) and part of the venous drainage (dashed white arrows) are obliterated by N-butylcyanoacrylate (NBCA). (h) The postembolization angiography (h) shows a complete obliteration of this compartment. (i) In the second session, the microcatheter (i; white arrows) is advanced distally to the posterolateral spinal artery (black arrow). (j) The fistula is obliterated by NBCA (j, white arrow). (k) The postembolization angiography (k) shows a complete obliteration of this compartment. (l) Finally, no further treatment is performed at the left T8 intercostal artery because no or minimal arteriovenous shunting is found. The patient experienced stability of symptoms at the 15-year follow-up.
DISCUSSION
In this report, we have presented our strategy for stratifying patients with PMAVF. We allocated patients to different groups, which received either surgical or endovascular treatment depending on the location of the lesion and the availability of endovascular materials.
We focused on the management strategy and classified PMAVF into three groups according to the location and size of the main feeder and the accessible treatment. Group 1 lesions, which involve dorsal PMAVFs, are more easily and directly approached using surgical treatment, whereas the fistula sites in Group 2 and Group 3 lesions are often difficult to locate, as they develop between congested veins, making localization and eradication of the shunt challenging.[
The key difference between the lesions in Groups 2 and 3 is the size of the dominant feeding artery. The diameter of the main arteries characterizes group 2 lesions according to the currently available smallest coil-deploying microcatheter (0.51–0.7 mm), such as the Excelsior SL-10 microcatheter (Stryker Neurovascular, USA) or Marathon microcatheter (Medtronic, Minneapolis, Minnesota, USA). This was done for two reasons. First, to date, the smallest microcatheter used in clinical practice for coil deployment has a diameter of approximately 1.5–1.7 F (0.51–0.68 mm). This is a limitation of the current technique, but the tool is available and suitable for our approach and planning in treating type 2 PMAVF lesions. Second, PMAVF lesions with a feeder larger than 0.7 mm are always associated with a large venous aneurysm close to the fistula; the endovascular technique is suitable in this case. With advances in microcatheter technology, even smaller microcatheters may be available for use in the future without changing this classification system.
Before Onyx was available, high concentrations (approximately 50%) of NBCA were used to treat brain AVM and AVFs using the “sandwich pushing technique” to manage fistulous components. However, the outcome was unpredictable due to the lack of control over the injection, which is a major concern when treating high-flow lesions, such as a PMAVF. One study by Hsu et al.[
Conversely, we also have the option of using Onyx, which can be injected slowly and in a repeated slow “push–pause–push” technique under fluoroscopic guidance. We do not have a preference between NBCA and Onyx, although some centers do not use Onyx for the treatment of PMAVF[
For Group 3 lesions, the embolization of multiple small feeders can only be accomplished using small microcatheters, and liquid embolic is the only suitable embolic material. Typically, 2 or 3 sessions are required for an acceptable result. However, the tortuosity and size of the feeding arteries in Group 3 PMAVFs pose challenges that make techniques such as the “pressure cooker” and the “double catheter” impractical for the embolization of PMAVFs.
Although postangiographic spontaneous closure of a PMAVF is rare, some studies have reported such closure.[
Previous studies on the endovascular treatment of PMAVFs have reported clinical improvements in 65–100% of patients, with various complete occlusion rates.[
This was a retrospective analysis of prospective data. Although the sample size was relatively small, PMAVF is a rare disease. A clinical trial comparing the outcomes of all reported techniques is impractical. Therefore, we proposed a simple strategy for managing PMAVF according to the fistula location and size of the dominant feeder. The key difference between Group 2 and Group 3 lesions is the size of the dominant feeder and the currently available smallest-sized microcatheter, which can deploy detachable coils and inject liquid embolic. The typical diameter of a microcatheter is approximately 0.7 mm. If microcatheters continue to trend smaller in the future, the definitions of these groups may be revised accordingly.
CONCLUSION
We proposed a simple but practical strategy for managing PMAVF according to the location of the fistula and the exact size of the dominant feeder. The strategy can be easily applied in clinical practice.
Ethical approval:
The research/study was approved by the Institutional Review Board at National Taiwan University Hospital, number 202005096RINB, dated May 26, 2020.
Declaration of patient consent:
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aydin K, Sencer S, Sencer A, Terzibasioglu E, Minareci O. Angiography-induced closure of perimedullary spinal arteriovenous fistula. Br J Radiol. 2004. 77: 969-73
2. Cho WS, Kim KJ, Kwon OK, Kim CH, Kim J, Han MH. Clinical features and treatment outcomes of the spinal arteriovenous fistulas and malformation: Clinical article. J Neurosurg Spine. 2013. 19: 207-16
3. Gupta V, Rizvi T, Garg A, Gaikwad SB, Mishra NK. Postangiographic thrombosis of a spinal arteriovenous malformation: Case report. J Neurosurg Spine. 2005. 2: 486-90
4. Horie N, So G, Debata A, Hayashi K, Morikawa M, Suyama K. Intra-arterial indocyanine green angiography in the management of spinal arteriovenous fistulae: Technical case reports. Spine (Phila Pa 1976). 2012. 37: E264-7
5. Hsiao IH, Lee HC, Yen PS, Cho DY. Embolization followed by surgery for treatment of perimedullary arteriovenous fistula causing acute myelopathy. Surg Neurol Int. 2015. 6: S275-8
6. Hsu YH, Lee CW, Liu HM, Wang YH, Kuo MF. Prioritized venous coiling facilitating endovascular treatment of brain arteriovenous malformations with a fistulous component. World Neurosurg. 2015. 84: 1857-63
7. Ji T, Guo Y, Shi L, Yu J. Study and therapeutic progress on spinal cord perimedullary arteriovenous fistulas. Biomed Rep. 2017. 7: 214-20
8. Kang J, Gregg L, Gailloud P. Spontaneous resolution of low-flow spinal arteriovenous fistulas. Neuroradiology. 2017. 59: 1003-12
9. Li J, Zeng G, Zhi X, Bian L, Yang F, Du J. Pediatric perimedullary arteriovenous fistula: Clinical features and endovascular treatments. J Neurointerv Surg. 2019. 11: 411-5
10. Liu HM, Wang YH, Chen YF, Tu YK, Huang KM. Endovascular treatment of brain-stem arteriovenous malformation: Safety and efficacy. Neuroradiology. 2003. 45: 644-9
11. Meng X, Zhang H, Wang Y, Ye M, He C, Du J. Perimedullary arteriovenous fistulas in pediatric patients: Clinical, angiographical, and therapeutic experiences in a series of 19 cases. Childs Nerv Syst. 2010. 26: 889-96
12. Merland JJ, Riche MC, Chiras J. Intraspinal extramedullary arteriovenous fistulae draining into the medullary veins. J Neuroradiol. 1980. 7: 271-320
13. Miyoshi Y, Yasuhara T, Nishida A, Tokunaga K, Sugiu K, Date I. Effectiveness of intraoperative near-infrared indocyanine green videoangiography in a case with recurrent spinal perimedullary arteriovenous fistula. Clin Neurol Neurosurg. 2011. 113: 239-42
14. Morgan MK, Chaseling R, Johnston I, De Silva M. Spinal arteriovenous malformation presenting at birth: Case report. Neurosurgery. 1986. 19: 637-40
15. Mourier KL, Gobin YP, George B, Lot G, Merland JJ. Intradural perimedullary arteriovenous fistulae: Results of surgical and endovascular treatment in a series of 35 cases. Neurosurgery. 1993. 32: 885-91
16. Narvid J, Hetts SW, Larsen D, Neuhaus J, Singh TP, McSwain H. Spinal dural arteriovenous fistulae: Clinical features and long-term results. Neurosurgery. 2008. 62: 159-66 discussion 166-7
17. Niimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal Dural arteriovenous fistulae: Results and follow-up. Neurosurgery. 1997. 40: 675-82 discussion 682-3
18. Panciani PP, Fontanella M, Crobeddu E, Schatlo B, Bergui M, Ducati A. Spontaneous occlusion of a spinal arteriovenous malformation: Is treatment always necessary. J Neurosurg Spine. 2010. 12: 397-401
19. Park JE, Koo HW, Liu H, Jung SC, Park D, Suh DC. Clinical characteristics and treatment outcomes of spinal arteriovenous malformations. Clin Neuroradiol. 2018. 28: 39-46
20. Phadke RV, Bhattacharyya A, Handique A, Jain K, Kumar A, Singh V. Endovascular treatment in spinal perimedullary arteriovenous fistula. Interv Neuroradiol. 2014. 20: 357-67
21. Rangel-Castilla L, Russin JJ, Zaidi HA, Martinez-Del-Campo E, Park MS, Albuquerque FC. Contemporary management of spinal AVFs and AVMs: Lessons learned from 110 cases. Neurosurg Focus. 2014. 37: E14
22. Roccatagliata L, Kominami S, Krajina A, Sellar R, Soderman M, Van Den Berg R. Spinal cord arteriovenous shunts of the ventral (anterior) sulcus: Anatomical, clinical, and therapeutic considerations. Neuroradiology. 2017. 59: 289-96
23. Rodesch G, Hurth M, Alvarez H, Tadie M, Lasjaunias P. Spinal cord intradural arteriovenous fistulae: Anatomic, clinical, and therapeutic considerations in a series of 32 consecutive patients seen between 1981 and 2000 with emphasis on endovascular therapy. Neurosurgery. 2005. 57: 973-83
24. Seki T, Hida K, Lee J, Yano S, Iwasaki Y. Intraoperative color Doppler sonography in the surgical treatment of perimedullary arteriovenous fistula--case report. Neurol Med Chir (Tokyo). 2005. 45: 100-3
25. Touho H, Monobe T, Ohnishi H, Karasawa J. Treatment of type II perimedullary arteriovenous fistulas by intraoperative transvenous embolization: Case report. Surg Neurol. 1995. 43: 491-6
26. Tsuruta W, Matsumaru Y, Miyachi S, Sakai N. Endovascular treatment of spinal vascular lesion in Japan: Japanese registry of neuroendovascular therapy (JR-NET) and JR-NET2. Neurol Med Chir (Tokyo). 2014. 54: 72-8
27. Trinh VT, Duckworth EA. Surgical excision of filum terminale arteriovenous fistulae after lumbar fusion: Value of indocyanine green and theory on origins (a technical note and report of two cases). Surg Neurol Int. 2011. 2: 63
28. Van Dijk JM, TerBrugge KG, Willinsky RA, Farb RI, Wallace MC. Multidisciplinary management of spinal Dural arteriovenous fistulas: Clinical presentation and long-term follow-up in 49 patients. Stroke. 2002. 33: 1578-83
29. Yamaguchi S, Hamamura T, Ito O, Sayama T, Shimogawa T, Matsukado K. Thoracocervical giant perimedullary arteriovenous fistula treated with transarterial embolization: A case report. No Shinkei Geka. 2013. 41: 247-53