- Department of Neurosurgery, Nil Ratan Sircar Medical College and Hospital, Kolkata, West Bengal, India
Correspondence Address:
Jiwesh Kumar, M.ch Resident, Department of Neurosurgery, Nil Ratan Sircar Medical College and Hospital, Kolkata, India
DOI:10.25259/SNI_598_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jiwesh Kumar, Kaushik Roy, Abhirup Chakraborty, Ritankar Patra. Tubercular arachnoiditis: A rare culprit of paraparesis in a young adult – A case report and review of literature. 22-Nov-2024;15:432
How to cite this URL: Jiwesh Kumar, Kaushik Roy, Abhirup Chakraborty, Ritankar Patra. Tubercular arachnoiditis: A rare culprit of paraparesis in a young adult – A case report and review of literature. 22-Nov-2024;15:432. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13241
Abstract
Background: The prevalence of central nervous system tuberculosis (TB) is about 1–2% of all TB cases. Atypical cases like the present case, being interpreted as leptomeningeal metastasis in magnetic resonance imaging (MRI), can pose a dilemma, delaying or even leading to mistreatment.
Case Description: A 19-year-old male presented with acute onset paraparesis and bowel bladder involvement presented with an MRI lumbar spine suggesting leptomeningeal metastasis from D11–L5 levels who underwent decompression biopsy which on histopathological examination revealed to be tubercular granulomatous infection. Anti-tubercular drug (ATD) started, and significant improvement was seen in the lower limb power and tone. The outcome of treatment has been unpredictable. Previous case studies having neurological deficits due to severe compression, including ours, show good recovery after surgical decompression and ATD regime.
Conclusion: Such cases should be managed with high suspicion as they can be easily misdiagnosed to be tumors, leading to mistreatment or delayed treatment.
Keywords: Arachnoiditis, Paraparesis, Radiculomyelopathy, Tuberculosis
INTRODUCTION
The prevalence of central nervous system tuberculosis (TB) is about 1–2% of all TB cases.[
CASE REPORT
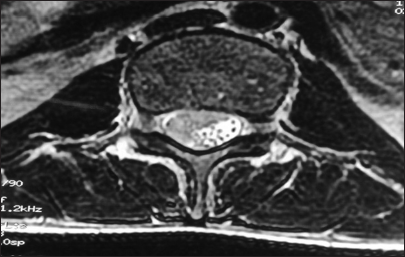
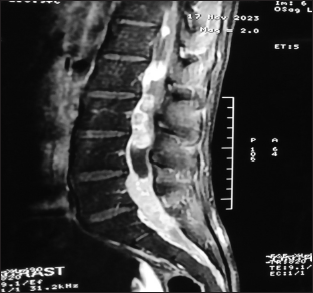
A 19-year-old young male student by occupation presented with dull, aching, progressively increasing back pain, radiating to both the lower limbs up to the foot for 4 months. This was accompanied by tingling and paresthesia of both the lower limbs. Three months later, he noticed the asymmetric onset of weakness in both the lower limbs involving proximal and distal muscles. He had developed numbness in the lower half of the body, including the perianal areas, only to progress to bladder disturbances, for which he used to give pressure in the suprapubic region to pass urine with constipation and erectile dysfunction for 2 weeks before admission. On clinical examination, he had asymmetric lower motor neuron type paraparesis with bladder and bowel involvement. Sensory loss (up to 50%) was present below the D12 dermatome, including the perianal region. Spinal tenderness and deformity were absent. He had a normal hemogram but an increased erythrocyte sedimentation rate (98 mm/1st h), being the only suspicious finding. X-rays of the lumbosacral spine and chest were normal. Contrast MRI (lumbosacral) revealed an ill-defined lesion in the lumbar region involving conus, with multiple leptomeningeal enhancements, in the intradural space at D12–S1, which was heterointence both in T1W and T2W [
He is able to stand and walk with support. Furthermore, he is able to pass urine voluntarily better than previously (he still has to give mild suprapubic pressure for complete evacuation of the bladder).
Unusual characteristics outlined in prior reports regarding spinal TB include (1) spinal posterior element involvement while sparing the anterior column, (2) occurrence of skip lesions, and (3) compression of neural components due to TB granuloma. The pedicle is the most frequently affected site within the posterior element of the spine. Intradural TB has been referred to by various terms, including intradural intramedullary/extramedullary TB, spinal arachnoiditis, and chronic adhesive arachnoiditis. It has been proposed to classify all these atypical TB forms under the term “tuberculous radiculomyelopathy” (TBRM).[
TBRM may develop from three different sources.
Primary TB lesion arising in the spinal meninges A downward extension from the intracranial TB meningitis A secondary spread from adjacent vertebrae disease.
Among these, downward spread from TBM is the most common. The thoracic cord is more frequently involved, followed by lumbar and cervical cords.
TBRM passes through three stages.[
Radiculitis–inflammation of pia arachnoid with associated hyperemia and swelling of roots. Arachnoiditis–progressive fibroblast proliferation and collagen deposition leading to nerve root adhesions to each other and pia arachnoid. Adhesive arachnoiditis–dense collagen deposition with encapsulation of atrophied nerve roots. Ours was in the second stage as per the features stated, extending from D11–L5, with densely adhered but not atrophied nerve roots. Furthermore, no other focus of TB, either in the brain or lungs could be ascertained.
The clinical manifestations are mostly either monoradicular or polyradicular pain syndromes. Rapidly evolving cases have been documented rarely as it usually takes several years.[
Classically, three MR patterns of arachnoiditis have been described involving cauda equina.[
Type 1: Central type–roots are clumped to the center of thecal sac Type 2: Peripheral type–roots are adherent to the margins of the dural sac Type 3: Adherent roots to one side of thecal sac resembling a soft-tissue mass.
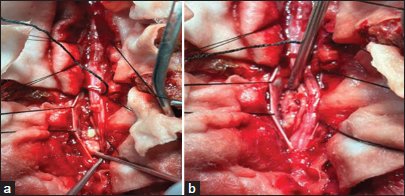
MRI of our case is of type 3, showing adherent roots filling the entire subarachnoid space resembling the conus cauda tumor. Differential diagnoses of contrast enhancing tumors in the conus cauda lesions in this age group include myxopapillary ependymoma, schwannoma, and paraganglioma. Also, to add to the dilemma were apparently normal intervertebral discs, and no pre/paravertebral collection/or any other inflammatory changes. Hence, all the findings never pointed to tubercular etiology. Before surgery, the dura was thick, roots of the cauda equina were adherent to each other and to the overlying dura. The roots of cauda equina were entangled in the granulation tissue. Similar intraoperative findings have been reported by Takahashi et al.,[
The management of TBRM primarily involves medical treatment, typically lasting 9–12 months. Surgical intervention should be considered only when HPE confirmation is necessary or in cases of spinal cord compression with neurological deficits or spinal instability. Treatment for spinal tuberculous arachnoiditis may involve either medical or surgical approaches, though medical treatment remains the cornerstone. Anti-TB therapy, using a combination of drugs, should be initiated once the diagnosis is confirmed.[
The outcome of treatment has been unpredictable, depending on the response of ATT on the individual patients; however, mostly favorable results are seen.[
CONCLUSION
Cases of tubercular arachnoiditis pose a great challenge to be diagnosed in the first look if there are no other foci of TB. Hence, such cases should be managed with high suspicion as they can be easily misdiagnosed to be tumors that may lead to mistreatment. MRI interpretation should be better, especially in endemic zones of TB, so that such cases are not missed, and collectively, we can contribute to achieving the TB eradication goal of the World Health Organization.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Burton CV. Lumbosacral arachnoiditis. Spine (Phila Pa 1976). 1978. 3: 24-30
2. Gourie-Devi M, Satishchandra P. Hyaluronidase as an adjuvant in the management of tuberculous spinal arachnoiditis. J Neurol Sci. 1991. 102: 105-11
3. Guleria R, Kavitha . Central nervous system tuberculosis. Indian J Tuberc. 2014. 61: 195-9
4. Hasegawa K, Murata H, Naitoh K, Nagano A. Spinal tuberculosis: Report of an atypical presentation. Clin Orthop Relat Res. 2002. 403: 100-103
5. Hoffman GS. Spinal arachnoiditis: What is the clinical spectrum?. Spine (Phila Pa 1976). 1983. 8: 538-40
6. Konar SK, Rao KN, Mahadevan A, Devi BI. Tuberculous lumbar arachnoiditis mimicking conus cauda tumor: A case report and review of literature. J Neurosci Rural Pract. 2011. 2: 93-6
7. Ross J, Masaryk T, Modic M, Delamater R, Bohlman H, Wilbur G. MR imaging of lumbar arachnoiditis. AJR Am J Roentgenol. 1987. 149: 1025-32
8. Sree Harsha CK, Shetty AP, Rajasekaran S. Intradural spinal tuberculosis in the absence of vertebral or meningeal tuberculosis: A case report. J Orthop Surg (Hong Kong). 2006. 14: 71-5
9. Takahashi H, Ito S, Kojima S, Tanno T, Hattori T. Intradural extramedullary tuberculoma of the thoracic spine: Paradoxical response to antituberculous therapy. Intern Med. 2008. 47: 797-8
10. Vidyasagar C, Murthy HK. Spinal tuberculosis with neurological deficits. Natl Med J India. 1996. 9: 25-7
11. Wadia NH, Dastur DK. Spinal meningitides with radiculomyelopathy: Clinical and radiological features. J Neurol Sci. 1969. 8: 239-60
12. WHO consolidated guidelines on tuberculos. Module 2: Screening-systematic screening for tuberculosis disease. Geneva: World Health Organization; 2021. p. Available from: https://www.who.int/publications/i/item/9789240022676 [Last accessed on 2024 Jul 20]