- Department of Neurosurgery, Chikamori Hospital, Kochi, Japan
- Department of Radiology, Chikamori Hospital, Kochi, Japan
- Department of Emergency and Critical Care Medicine, Chikamori Hospital, Kochi, Japan
Correspondence Address:
Satoru Hayashi
Department of Emergency and Critical Care Medicine, Chikamori Hospital, Kochi, Japan
DOI:10.4103/sni.sni_129_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Satoru Hayashi, Koji Hosoda, Yo Nishimoto, Motonobu Nonaka, Shinya Higuchi, Toshifumi Miki, Masatoshi Negishi. Unexpected intraabdominal hemorrhage due to segmental arterial mediolysis following subarachnoid hemorrhage: A case of ruptured intracranial and intraabdominal aneurysms. 29-Aug-2018;9:175
How to cite this URL: Satoru Hayashi, Koji Hosoda, Yo Nishimoto, Motonobu Nonaka, Shinya Higuchi, Toshifumi Miki, Masatoshi Negishi. Unexpected intraabdominal hemorrhage due to segmental arterial mediolysis following subarachnoid hemorrhage: A case of ruptured intracranial and intraabdominal aneurysms. 29-Aug-2018;9:175. Available from: http://surgicalneurologyint.com/surgicalint-articles/8996/
Abstract
Background:Segmental arterial mediolysis (SAM) is an uncommon vascular disease, which manifests as catastrophic intraabdominal hemorrhage caused by rupture of visceral dissecting aneurysms in most cases. The etiology of SAM is still unclear, but SAM may be a vasospastic disorder and the responsible pressor agent is norepinephrine. Recently, abdominal SAM coexisting with intracranial dissecting aneurysms has been reported, but the relationship between intraabdominal and intracranial aneurysms in SAM remains unclear, as no cases of concomitant abdominal SAM and ruptured intracranial saccular aneurysm have been reported.
Case Description:A 49-year-old woman underwent emergent clipping for a ruptured saccular aneurysm at the left C1 portion of the internal carotid artery. Intraoperatively, norepinephrine was continuously administered intravenously under general anesthesia. Four days after the subarachnoid hemorrhage (SAH), the patient suddenly developed shock due to massive hematoma in the abdominal cavity. Imaging showed multiple aneurysms involving the splenic artery, gastroduodenal artery, common hepatic artery, and superior mesenteric artery. Coil embolization of the splenic artery was performed immediately to prevent bleeding. Subsequent treatment for cerebral vasospasm following SAH was performed with prevention of hypertension, and the patient recovered with left temporal lobe infarction. The diagnosis was abdominal SAM based on the clinical, imaging, and laboratory findings.
Conclusion:Norepinephrine release induced by SAH and/or iatrogenic administration of norepinephrine may have promoted abdominal SAM in this case. Abdominal SAM may occur subsequent to rupture of ordinary saccular aneurysm, and may provoke catastrophic abdominal hemorrhage in the spasm stage after SAH.
Keywords: Internal carotid artery, intraabdominal aneurysm, norepinephrine, segmental arterial mediolysis, subarachnoid hemorrhage
INTRODUCTION
Segmental arterial mediolysis (SAM) is an uncommon nonatherosclerotic, noninflammatory vascular disease.[
CASE REPORT
A 49-year-old woman consulted our hospital because of sudden onset of headache and vomiting. Physical examination found the World Federation of Neurosurgical Societies grade II with systolic blood pressure of 152 mm Hg. No morphological features were identified suggesting congenital structural vascular disease such as Ehlers–Danlos syndrome or Marfan's syndrome. Her past history and family history were unremarkable, and systemic evaluation demonstrated no abnormality. Most laboratory data on admission were unremarkable including white blood cell count of 11.3 × 103/mm3, C-reactive protein level of 0.3 mg/dL, and negative findings for human immunodeficiency virus and syphilis. She smoked 10 cigarettes per day and occasionally consumed alcohol. Computed tomography (CT) of the head revealed SAH [
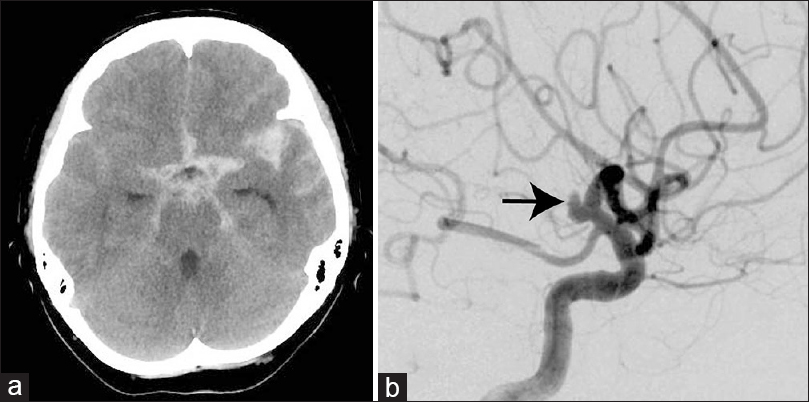
Figure 1
(a) Computed tomography scan of the head demonstrating subarachnoid hemorrhage mainly in the left sylvian fissure. (b) Left carotid angiogram showing a saccular aneurysm (arrow) at the C1 portion of the left internal carotid artery without the typical angiographic appearance of dissecting aneurysm such as focal irregularity of the vessel wall and fusiform dilatation
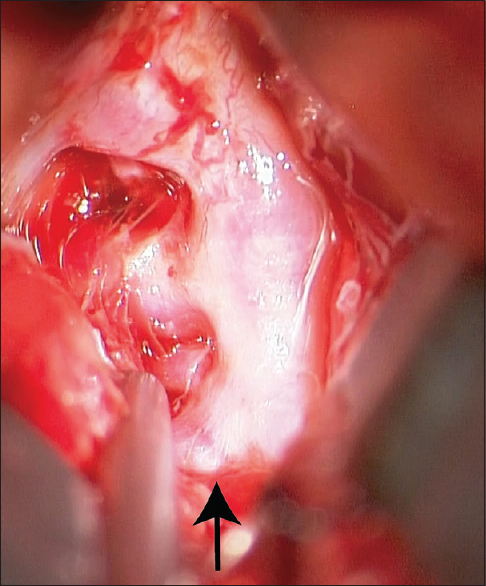
The patient underwent emergent clipping of the typical saccular aneurysm which had no features of dissection [
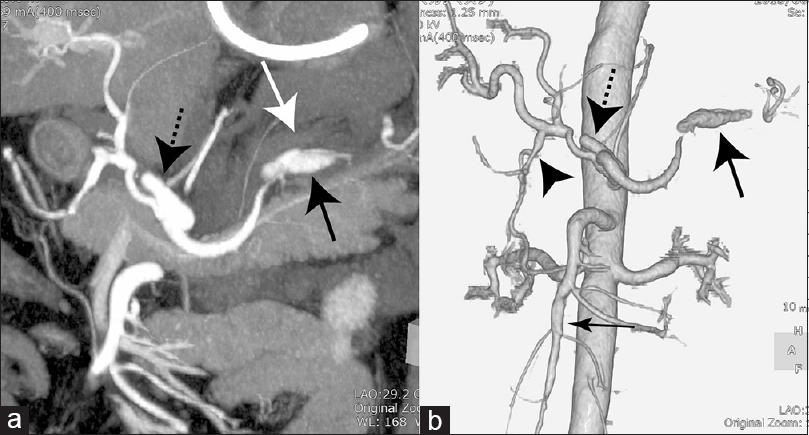
Figure 3
(a) Oblique coronal maximum intensity projection image of the abdomen demonstrating intraabdominal hematoma (white arrow) around the splenic artery aneurysm (black arrow) and common hepatic artery aneurysm (dotted arrow). (b) Three-dimensional computed tomography angiogram of the abdomen showing multiple aneurysms with the characteristics of dissection of the splenic artery (large arrow), gastroduodenal artery (arrowhead), common hepatic artery (dotted arrow), and superior mesenteric artery (small arrow)
Treatment for cerebral vasospasm following SAH was performed with prevention of hypertension, which might be contraindicated in the presence of abdominal lesions. She was given oral and continuous intravenous administration of calcium antagonist to maintain a systolic blood pressure of 100–140 mm Hg. Left temporal lobe infarction was detected, but the patient was discharged after 1 month hospitalization with mild aphasia which recovered completely afterward. The patient remained asymptomatic for 34 months after SAH and CT angiography of the head and abdomen demonstrated no recurrence of the aneurysms in both visceral and intracranial arteries.
DISCUSSION
The differential diagnosis of SAM includes atherosclerosis, fibromuscular dysplasia (FMD), Behçet's disease, polyarteritis nodosa, neurofibromatosis, Ehlers–Danlos syndrome type IV, Marfan's syndrome, and mycotic aneurysms.[
SAM was first described as a distinct pathological entity in 1976, [
Recently, abdominal SAM coexisting with intracranial dissecting aneurysms has been reported. Locations of the intracranial aneurysm included the ICA, basilar artery, vertebral artery (VA), and anterior cerebral artery.[
The intracranial aneurysm of our patient seemed not to be related to SAM in contrast to the abdominal lesions because of the absence of dissection, but occurred as a typical saccular aneurysm. The limitation of this case is the absence of histopathological evidence of abdominal SAM, but it is important to be aware of the possibility that abdominal SAM may occur subsequent to rupture of ordinary saccular aneurysm, and may provoke catastrophic abdominal hemorrhage in the spasm stage after SAH. The different etiologies of the intracranial and intraabdominal aneurysms in this case may indicate that intracranial dissecting aneurysms coexisting with abdominal SAM are not necessarily caused by SAM and the concomitance of abdominal SAM and SAH is not rare.
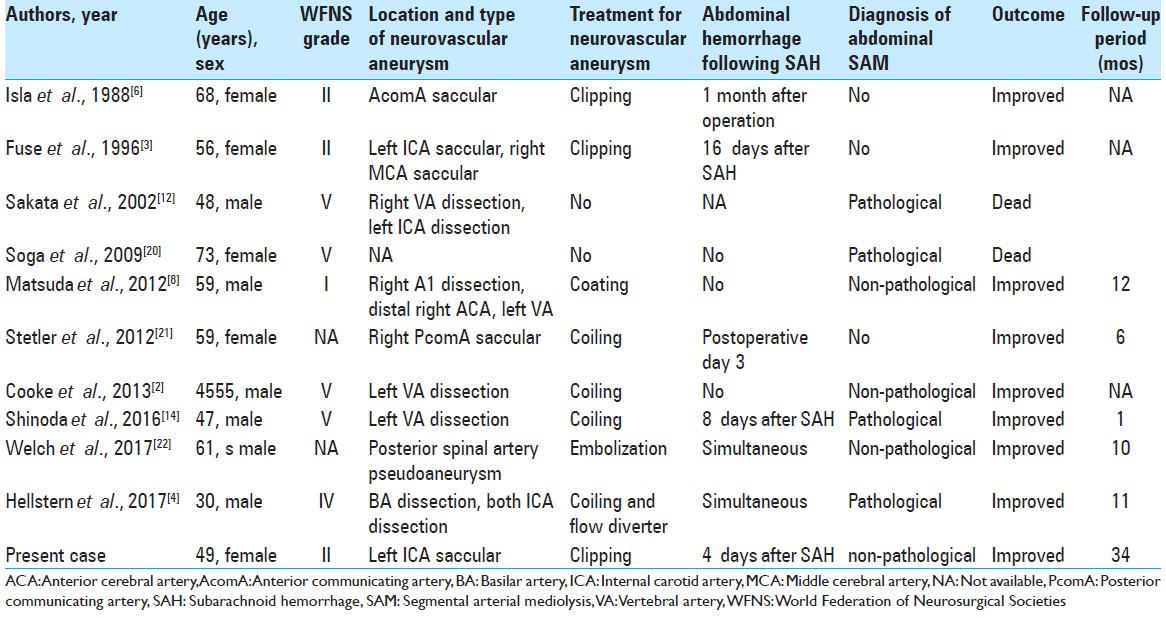
Ten cases of coexisting SAH and visceral aneurysm have been reported.[
The postoperative hypertensive state to counteract cerebral vasospasm following SAH may have also influenced the abdominal bleeding of SAM in this case, but the ideal management strategies for vasospasm in the presence of abdominal SAM have not yet been established because of the rarity of SAM following SAH. The catastrophic abdominal bleeding due to SAM during the acute stage of SAH prevents safe treatment for vasospasm following SAH, so blood transfusion and hemostasis of abdominal bleeding should be ensured immediately as the first step for such hemorrhagic shock. Moreover, it is important to recognize the causative abdominal lesions as SAM, because the resultant shock should not be treated by adrenergic agents which could intensify SAM and generate additional lesions.[
CONCLUSION
Norepinephrine release induced by SAH and/or iatrogenic administration of norepinephrine may have promoted abdominal SAM in this case. Little is known about the association of increased levels of norepinephrine with SAM after SAH, in spite of the risk that SAM may induce sudden visceral hemorrhage in the unstable cerebral circulation stage of vasospasm, which is likely to result in death and needs prompt diagnosis and treatment. Neurosurgeons should be aware of the possibility of unexpected abdominal hemorrhage caused by SAM within several days following SAH. Early recognition of SAM and management can lead to significant reduction in subsequent complications and mortality. The different etiologies of the intracranial and intraabdominal aneurysms in this case may indicate that intracranial dissecting aneurysms coexisting with abdominal SAM are not necessarily caused by SAM and the concomitance of abdominal SAM and SAH is not rare. The natural history of SAM is poorly understood, so we continue to follow up our patients regularly.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. The form specifies that the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baker-LePain JC, Stone DH, Mattis AN, Nakamura MC, Fye KH. Clinical diagnosis of segmental arterial mediolysis: Differentiation from vasculitis and other mimics. Arthritis Care Res (Hoboken). 2010. 62: 1655-60
2. Cooke DL, Meisel KM, Kim WT, Stout CE, Halbach VV, Dowd CF. Serial angiographic appearance of segmental arterial mediolysis manifesting as vertebral, internal mammary and intra-abdominal visceral artery aneurysms in a patient presenting with subarachnoid hemorrhage and review of the literature. J Neurointerv Surg. 2013. 5: 478-82
3. Fuse T, Takagi T, Yamada K, Fukushima T. Systemic multiple aneurysms of the intracranial arteries and visceral arteries: Case report. Surg Neurol. 1996. 46: 258-61
4. Hellstern V, Aguilar Pérez M, Kohlhof-Meinecke P, Bäzner H, Ganslandt O, Henkes H. Concomitant retroperitoneal and subarachnoid hemorrhage due to segmental arterial mediolysis: Case report and review of the literature. Clin Neuroradiol. 2018. 28: 445-50
5. Inada K, Maeda M, Ikeda T. Segmental arterial mediolysis: Unrecognized cases culled from cases of ruptured aneurysm of abdominal visceral arteries reported in the Japanese literature. Pathol Res Pract. 2007. 203: 771-8
6. Isla A, Roda JM, Alvarez F, Blázquez MG. Concurrent intracranial and intraabdominal aneurysms. J Neurosurg Sci. 1988. 32: 121-2
7. Kalva SP, Somarouthu B, Jaff MR, Wicky S. Segmental arterial mediolysis: Clinical and imaging features at presentation and during follow-up. J Vasc Interv Radiol. 2011. 22: 1380-7
8. Matsuda R, Hironaka Y, Takeshima Y, Park YS, Nakase H. Subarachnoid hemorrhage in a case of segmental arterial mediolysis with coexisting intracranial and intraabdominal aneurysms. J Neurosurg. 2012. 116: 948-51
9. Naredi S, Lambert G, Edén E, Zäll S, Runnerstam M, Rydenhag B. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000. 31: 901-6
10. Ro A, Kageyama N, Takatsu A, Fukunaga T. Segmental arterial mediolysis of varying phases affecting both the intra-abdominal and intracranial vertebral arteries: An autopsy case report. Cardiovasc Pathol. 2010. 19: 248-51
11. Ro A, Kageyama N. Pathomorphometry of ruptured intracranial vertebral arterial dissection: Adventitial rupture, dilated lesion, intimal tear, and medial defect. J Neurosurg. 2013. 119: 221-7
12. Sakata N, Takebayashi S, Shimizu K, Kojima M, Masawa N, Suzuki K. A case of segmental mediolytic arteriopathy involving both intracranial and intraabdominal arteries. Pathol Res Pract. 2002. 198: 493-7
13. Shenouda M, Riga C, Naji Y, Renton S. Segmental arterial mediolysis: A systematic review of 85 cases. Ann Vasc Surg. 2014. 28: 269-77
14. Shinoda N, Hirai O, Mikami K, Bando T, Shimo D, Kuroyama T. Segmental arterial mediolysis involving both vertebral and middle colic arteries leading to subarachnoid and intraperitoneal hemorrhage. World Neurosurg. 2016. 88: 694e5-10
15. Slavin RE, Gonzalez-Vitale JC. Segmental mediolytic arteritis: A clinical pathologic study. Lab Invest. 1976. 35: 23-9
16. Slavin RE, Cafferty L, Cartwright J. Segmental mediolytic arteritis. A clinicopathologic and ultrastructural study of two cases. Am J Surg Pathol. 1989. 13: 558-68
17. Slavin RE, Yaeger MJ. Segmental arterial mediolysis – An iatrogenic vascular disorder induced by ractopamine. Cardiovasc Pathol. 2012. 21: 334-8
18. Slavin RE. Segmental arterial mediolysis: A clinical-pathologic review, its role in fibromuscular dysplasia and description and differential diagnosis of the masquerader-muscular artery cystic necrosis. World J Cardiovasc Dis. 2013. 3: 64-81
19. Slavin RE. Segmental arterial mediolysis: A review of a proposed vascular disease of the peripheral sympathetic nervous system – A density disorder of the alpha-1 adrenergic receptor?. J Cardiovasc Dis Diagn. 2015. 3: 190-
20. Soga Y, Nose M, Arita N, Komori H, Miyazaki T, Maeda T. Aneurysms of the renal arteries associated with segmental arterial mediolysis in a case of polyarteritis nodosa. Pathol Int. 2009. 59: 197-200
21. Stetler WR, Pandey AS, Mashour GA. Intracranial aneurysm with concomitant rupture of an undiagnosed visceral artery aneurysm. Neurocrit Care. 2012. 16: 154-7
22. Welch BT, Brinjikji W, Stockland AH, Lanzino G. Subarachnoid and intraperitoneal hemorrhage secondary to segmental arterial mediolysis: A case report and review of the literature. Interv Neuroradiol. 2017. 23: 378-81