- Department of Radiology, Rutgers Robert Wood Johnson Medical School, New Brunswick, United States
- Department of Neurosurgery, Rutgers Robert Wood Johnson Medical School, New Brunswick, United States
Correspondence Address:
Stephen Joseph Sozio, Department of Radiology, Rutgers Robert Wood Johnson Medical School, New Brunswick, United States. ;
DOI:10.25259/SNI_1062_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Muhammad Omar Afridi1, Stephen Joseph Sozio1, Sudipta Roychowdhury1, Gaurav Gupta2, Emad Nourollah-Zadeh2, Hai Sun2, Arevik Abramyan2, Sri Hari Sundararajan1. Use of flow-diverting stents in the treatment of ruptured intracranial artery dissections. 09-May-2025;16:172
How to cite this URL: Muhammad Omar Afridi1, Stephen Joseph Sozio1, Sudipta Roychowdhury1, Gaurav Gupta2, Emad Nourollah-Zadeh2, Hai Sun2, Arevik Abramyan2, Sri Hari Sundararajan1. Use of flow-diverting stents in the treatment of ruptured intracranial artery dissections. 09-May-2025;16:172. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13547
Abstract
Background: Flow-diverting stents have rapidly become widespread as the preferred treatment for intracranial aneurysms. They function by optimizing blood flow within the parent vessel to reduce shear stress in the aneurysm, facilitating thrombus formation within the aneurysm, and providing a scaffold for endothelialization and vascular remodeling. Early literature indicates this mechanism also applies to the treatment of ruptured intracranial aneurysms and unruptured intracranial artery dissections, demonstrating favorable occlusion rates. We highlight a novel application of flow-diverting stents in the treatment of ruptured intracranial artery dissections.
Case Description: A retrospective chart review was performed of two adult patients suffering from ruptured middle cerebral artery dissections, in which a Flow Re-direction Endoluminal Device X (FRED X stent, Microvention™, USA) was deployed across the dissection. Utilizing both the flow diverting and vascular remodeling properties of the FRED X stent, both patients achieved hemostasis, maintenance intraluminal patency, and ultimately resolution of hemorrhage without any treatment-related complications. Short-term follow-up revealed no bleeding recurrence.
Conclusion: Flow-diverting stents show promise as a viable option for managing ruptured intracranial artery dissections. However, further prospective studies are recommended to evaluate their efficacy and long-term outcomes comprehensively.
Keywords: Dissection, Endovascular procedures, Interventional radiology, Intracranial arterial diseases, Stents
INTRODUCTION
The introduction of flow-diversion technology has revolutionized the approach to treating intracranial aneurysms following its Food and Drug Administration approval. Flow-diverting stents aim to optimize blood flow within the parent vessel, redirecting it away from the aneurysm sac. Simultaneously, they serve as a scaffold to facilitate endothelialization and promote vascular remodeling to heal the wall defect causing aneurysmal dilation. The mechanism of flow-diverting stents can be divided into three phases: hemodynamic, thrombus formation, and endothelialization. The hemodynamic phase occurs immediately as the low-porosity stent is deployed across the aneurysm inflow site, significantly reducing the hemodynamic velocity of blood flow and shear stress within the aneurysm.[
The initial Pipeline for Uncoilable or Failed Aneurysms trial was one of the earliest studies advocating for the effectiveness of flow-diverting stents. This trial demonstrated successful placement of flow-diverting stents in 107 out of 108 unruptured, large, and wide-necked aneurysms (99.1%), achieving an 86.8% complete aneurysm occlusion rate at the 1-year follow-up. Notably, major complications, such as major ipsilateral stroke and neurological death, occurred in 6 patients (5.6%).[
To our knowledge, studies evaluating the use of the flow-diverting stents for the treatment of ruptured intracranial arterial dissection, specifically the Flow Re-direction Endoluminal Device X (FRED X stent, Microvention™, USA), have not been well-documented. Here, we present two cases wherein ruptured middle cerebral artery (MCA) dissections were successfully treated using a FRED X flow-diverting stent, achieving medical stability with no rebleeding or other postoperative complications.
CASE REPORT
Case 1
A patient of 60–70 years old with a past medical history of atrial fibrillation on chronic anticoagulation, mitral valve replacement, hypertension, hyperlipidemia, and type 2 diabetes mellitus presented to the emergency department with left-sided facial droop, left arm and leg weakness, and slurred speech. Noncontrast computed tomography (CT) of the head revealed a dense proximal right MCA, with suspected calcific thrombus within the M1 segment. Subsequent contrast-enhanced CT angiography (CTA) of the head illustrated acute, focal occlusion of the right M1 segment with attenuated downstream reconstitution of flow [
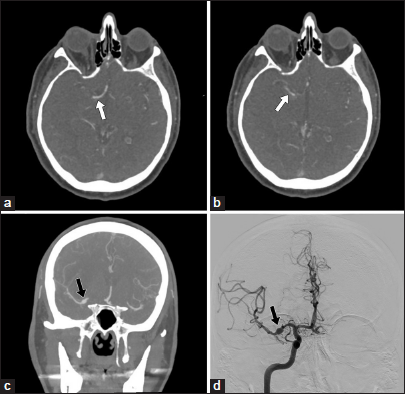
Figure 1:
(a and b) Initial computed tomography angiography (CTA) of the head demonstrates an acute focal occlusion of the M1 segment of the right middle cerebral artery with attenuated distal reconstitution of flow (white arrows); (c) CTA of the head showing 5.2 mm fusiform dilation of the right M1 (black arrow) at the site of the mechanical thrombectomy performed 8 days prior; (d) Right internal carotid artery angiogram confirming the presence of a 5.2 mm fusiform aneurysm at the right M1 segment, compatible with a pseudoaneurysm secondary to an M1 dissection (black arrow).
While the patient remained asymptomatic following the procedure, a 24-hour follow-up CT of the head revealed hyperdensity in the right Sylvian fissure, indicating either residual contrast staining or subarachnoid blood products. Subsequent noncontrast magnetic resonance imaging (MRI) confirmed hemorrhagic infarction (HI) type 2 (HI-2) parenchymal hemorrhage within the posterior and medial aspects of the evolving infarction. The decision was made to manage this bleeding medically. Additionally, concerning findings for prosthetic endocarditis emerged during transesophageal echocardiography. A head CTA was conducted to assess for a potential mycotic aneurysm. The resulting CTA [
A repeat CTA of the head [
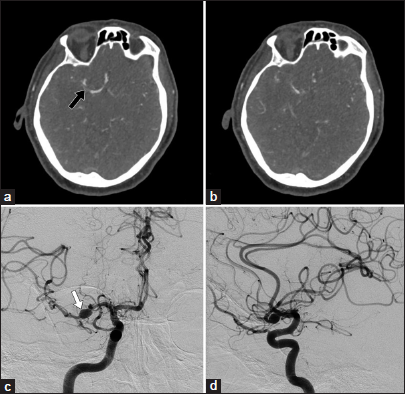
Figure 2:
(a and b) Repeat computed tomography angiography demonstrating minimal growth of the pseudoaneurysm, with increased luminal narrowing at the right M1 segment just distal to the pseudoaneurysm (black arrow); (c) Frontal view right internal carotid artery (ICA) angiogram illustrating an enlarging middle cerebral artery (MCA) pseudoaneurysm (white arrow); (d) Additional lateral view of the right ICA angiogram confirms an enlarging MCA pseudoaneurysm.
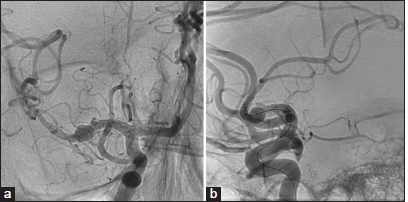
The patient underwent successful and uncomplicated embolization of the right MCA ruptured dissection/ pseudoaneurysm using the FRED X stent. Subsequent imaging showed promising results: a 1-month follow-up brain MRI revealed a decreasing size of the intracranial hemorrhage, which the 6-month follow-up CT of the head completely resolved. In addition, a 6-month diagnostic angiogram [
Of note, this patient received standard protocol oral antiplatelet therapy utilizing aspirin 81 mg daily and ticagrelor 90 mg twice daily for the initial 5 months following stenting; this was then discontinued as the patient required mitral valve replacement, at which time both agents were stopped 5 days before the replacement. Following this procedure, the patient received clopidogrel 75 mg daily, which was ultimately discontinued at 9 months after stenting.
Case 2
A patient of 70–80 years old with a medical history of atrial fibrillation, an ischemic stroke 6 years ago without residual deficits, hypertension, and hyperlipidemia presented to the emergency department with right-sided flaccid paralysis, difficulty with rightward gaze, and dysarthria. An emergent noncontrast CT of the head revealed a dense left MCA and loss of gray-white differentiation within the left insular ribbon and mesial temporal lobe, consistent with an acute ischemic infarct. Subsequent contrast-enhanced CTA of the head confirmed extensive occlusion of the left internal carotid artery terminus with the extension of the thrombus into the proximal left A1 and proximal-to-mid left M1 segments, with additional distal occlusions of the distal left M1/proximal M2 segments with poor distal vascular reconstitution [
Figure 4:
(a) Initial computed tomography angiography of the head illustrating occlusive thrombus involving the proximal-to-mid left M1 segments with poor distal vascular reconstitution (white arrow); (b) Left internal carotid artery (ICA) angiogram confirming occlusive thrombus within the left M1 segment (black arrow) before mechanical thrombectomy; (c) Left ICA angiogram confirming distal reconstitution of flow (black arrow) after mechanical thrombectomy.
Mechanical thrombectomy was pursued, involving three attempts at reperfusion: two unsuccessful passes were made using the Red 68 aspiration catheter (Penumbra™, USA) with Trevo 6 × 37 mm Stent Retriever (Stryker™, USA), followed by a final successful pass using the Zoom 71 aspiration catheter (Imperative Care™, USA) with Zoom 88 Sheath Relay (Imperative Care™, USA). This final attempt achieved the reconstitution of flow within the occluded M1 segment, resulting in a TICI score of 3 [
Seven days after the left M1 thrombectomy, the patient developed sudden right-sided weakness and right facial droop. A subsequent CT of the head revealed a new subarachnoid hemorrhage within the left Sylvian fissure and a small left inferior frontal intraparenchymal hematoma [
Figure 5:
(a) Computed tomography (CT) of the head 7 days after mechanical thrombectomy demonstrating a new left inferior frontal intraparenchymal hemorrhage and a small left Sylvian fissure subarachnoid hemorrhage (white arrow); (b) Left internal carotid artery (ICA) cerebral angiogram performed 7 days after mechanical thrombectomy showing luminal narrowing of the left M1 segment (black arrow), compatible with a dissection flap; (c) Poststenting CT angiography of the head demonstrating stent placement (white arrow); (d) Left ICA cerebral angiogram confirms a patent left MCA (black arrow) with moderate in-stent stenosis (the angiogram was performed after treatment with intraarterial Integrilin).
Unfortunately, a CTA of the head immediately after the procedure revealed a new thrombus within the distal aspect of the stent, with mild reconstitution of flow to the parasylvian branches of the left MCA [
DISCUSSION
Unstable intracranial artery dissections with luminal narrowing, predominantly located in the distal segments of the internal carotid artery, are typically managed using highly flexible stents with moderate radial force, such as the Neuroform Atlas stent (Stryker™, USA).[
Flow-diverting stents have demonstrated significant effectiveness in the treatment of intracranial arterial aneurysms. Early data also indicate promising results in managing ruptured aneurysms. A systematic review and meta-analysis conducted by Cagnazzo et al., which included 20 studies and a total of 223 patients with acutely ruptured intracranial aneurysms treated with flow-diverting stents, revealed that 32% achieved immediate angiographic occlusion, with 88.9% achieving long-term complete/ near-complete aneurysm occlusion. The treatment-related complication rate was 17.8%, and the aneurysm rebleeding rate posttreatment was 4%, with a higher likelihood of rebleeding within the first 72 h.[
We utilized both the flow-diverting and vascular remodeling properties of the FRED X stent to treat ruptured intracranial artery dissections. In the first case, we successfully treated a ruptured right M1 dissecting pseudoaneurysm, and in the second case, we successfully treated a ruptured distal left M1 dissection. Neither patient experienced any treatment-related complication or rebleeding. A 6-month follow-up angiogram for the first case confirmed a stable stent position and a slight decrease in size of the right M1 pseudoaneurysm, with no evidence of rebleeding. In the second case, a same-day angiogram demonstrated a patent left MCA poststenting without rebleeding or recurrent symptoms. A 3-month follow-up CTA of the head confirmed stable postsurgical changes with a patent stent and no signs of intracranial hemorrhage. These favorable outcomes, coupled with the absence of procedural complications in both immediate and 6-month follow-ups, support the efficacy of the FRED X stent in treating ruptured intracranial artery dissections.
CONCLUSION
Previous data have shown the effectiveness of flow-diverting stents for the management of ruptured intracranial aneurysms and unruptured intracranial artery dissections. We present two cases that support the utilization of flow-diverting stents in the management of contained ruptured intracranial artery dissections. Likely, the same mechanisms in which these stents are effective for ruptured aneurysms and unruptured dissections can also be applied to ruptured dissections. By facilitating the natural healing response of the vessel wall and providing a scaffold, the stent aims to limit the extraluminal blood extravasation through its poorly porous wall. Given the small sample size and retrospective nature of this work, future prospective studies are recommended to assess their efficacy and long-term outcomes comprehensively. Evaluating the use of flow-diverting stents in managing ruptured intracranial arterial dissections would provide vital insight into their potential effectiveness and assist in refining treatment strategies.
Ethical approval:
Institutional Review Board approval is not required given the retrospective nature of this study.
Declaration of patient consent:
Patient consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aenis M, Stancampiano AP, Wakhloo AK, Lieber BB. Modeling of flow in a straight stented and nonstented side wall aneurysm model. J Biomech Eng. 1997. 119: 206-12
2. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology. 2013. 267: 858-68
3. Borota L, Mahmoud E, Nyberg C. Neuroform Atlas stent in treatment of iatrogenic dissections of extracranial internal carotid and vertebral arteries: A single-centre experience. Interv Neuroradiol. 2019. 25: 390-6
4. Cagnazzo F, Lefevre PH, Derraz I, Dargazanli C, Gascou G, Di Carlo DT. Flow-diversion treatment for unruptured nonsaccular intracranial aneurysms of the posterior and distal anterior circulation: A meta-analysis. AJNR Am J Neuroradiol. 2020. 41: 134-9
5. Dandapat S, Mendez-Ruiz A, Martínez-Galdámez M, Macho J, Derakhshani S, Foa Torres G. Review of current intracranial aneurysm flow diversion technology and clinical use. J Neurointerv Surg. 2021. 13: 54-62
6. Mpotsaris A, Skalej M, Beuing O, Eckert B, Behme D, Weber W. Long-term occlusion results with SILK flow diversion in 28 aneurysms: Do recanalizations occur during follow-up?. Interv Neuroradiol. 2015. 21: 300-10
7. Schirmer CM, Bulsara KR, Al-Mufti F, Haranhalli N, Thibault L, Hetts SW. Antiplatelets and antithrombotics in neurointerventional procedures: Guideline update. J Neurointerv Surg. 2023. 15: 1155-62
8. Shehata MA, Ibrahim MK, Ghozy S, Bilgin C, Jabal MS, Kadirvel R. Long-term outcomes of flow diversion for unruptured intracranial aneurysms: A systematic review and meta-analysis. J Neurointerv Surg. 2023. 15: 898-902
9. Suh DC, Song Y, Park SI, Kwon B. Flow Diverter treatment using a flow re-direction endoluminal device for unruptured intracranial vertebral artery dissecting aneurysm: Single-center case series and technical considerations. Neurointervention. 2023. 18: 114-22
10. Sweid A, Rahm SP, Das S, Baldassari MP, Jabbour P, Alexander TD. Safety and efficacy of bilateral flow diversion for treatment of anterior circulation cerebral aneurysms. World Neurosurg. 2019. 130: e1116-21
11. Walcott BP, Stapleton CJ, Choudhri O, Patel AB. Flow diversion for the treatment of intracranial aneurysms. JAMA Neurol. 2016. 73: 1002-8