- Department of Neurosurgery, Keio University, School of Medicine, Shinjuku, Japan.
Correspondence Address:
Katsuhiro Mizutani, Department of Neurosurgery, Keio University, School of Medicine, Shinjuku, Japan.
DOI:10.25259/SNI_214_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kosei Yamamoto, Katsuhiro Mizutani, Takenori Akiyama, Hirotsugu Nogawa, Masahiro Toda. Vasa vasorum: The role in intracranial physiology and pathophysiology. 07-Jun-2024;15:188
How to cite this URL: Kosei Yamamoto, Katsuhiro Mizutani, Takenori Akiyama, Hirotsugu Nogawa, Masahiro Toda. Vasa vasorum: The role in intracranial physiology and pathophysiology. 07-Jun-2024;15:188. Available from: https://surgicalneurologyint.com/surgicalint-articles/12934/
Abstract
Background: Vasa vasorum (VVs) is a Latin word representing vessels of vessels. VVs are usually found on the adventitia of the parent vessel and infrequently reach the media and intima, depending on the size and type of the parent vessels and physiological and pathological conditions. The VVs include arteries, capillaries, veins, and lymphatic vessels, involving the oxygenation and nourishment of the vessel’s wall to sustain its healthy state. Accumulated studies have revealed that VVs are involved in various intracranial lesions, including atherosclerotic diseases, aneurysms, and shunt diseases. The current review aims to review and integrate past and recent findings and knowledge on VVs and to facilitate our understanding of VVs and intracranial pathology involving VVs.
Methods: A literature review was carried out with a focus on the role of VVs by searching the Pubmed database.
Results: We identified 71 articles that discuss the role of VVs. We discussed the anatomical structure, physiological significance, and pathological significance of the VV.
Conclusion: VV is not only involved in the nutrition and metabolism of the vascular wall but is also deeply involved in the pathogenesis of inflammation, ischemia, and thrombosis of the vascular wall. In addition, in the central nervous system, intracranial vascular wall nutrient particularities and VVs are closely related to the pathogenesis of cerebral aneurysms, subarachnoid hemorrhage, arteriovenous shunt disease, atherosclerotic lesions, and other conditions.
Keywords: Cerebral aneurysms, Intracranial vessels, Vasa vasorum
INTRODUCTION (HISTORY OF VASA VASORUM [VV])
VVs are a Latin word representing vessels of vessels. It is also called vasa nutria [
BASIC HISTOLOGICAL ANATOMY OF BLOOD VESSELS
The vessel wall comprises the tunica intima, media, and adventitia[
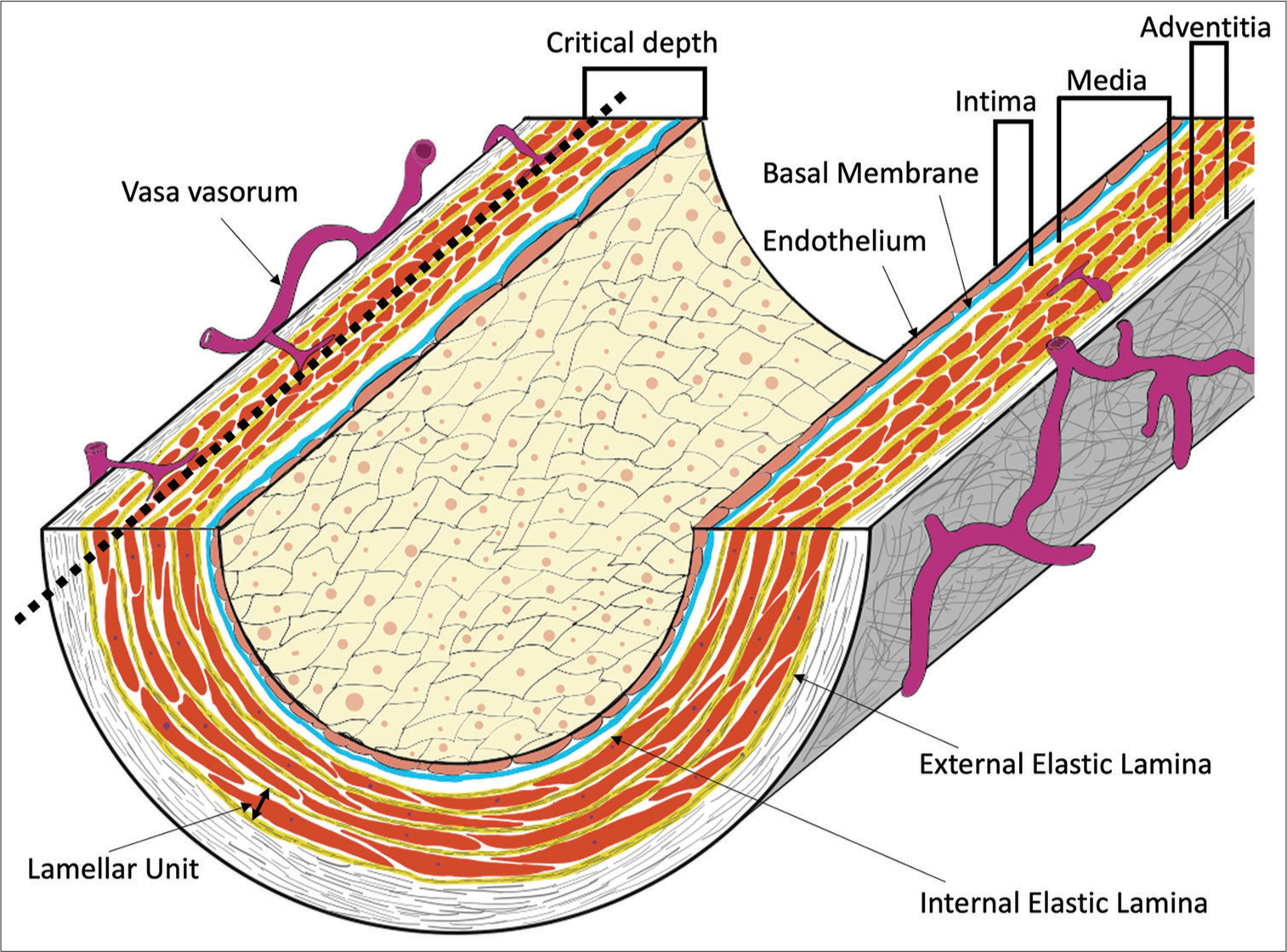
Figure 1:
The basic structure of a blood vessel (elastic artery) and vasa vasorum (VV). Elastic arteries have a well-developed elastic plate and smooth muscle (lamellar unit). Critical depth is considered to be the limit of oxygen and nutrients from the lumen, and VVs exist on the outer side of the lumen. The critical depth is usually approximately 0.5 mm (30 layers in a lamellar unit). Muscular arteries do not have such a well-developed lamellar unit. In intracranial arteries, the external elastic lamina is absent.
ANATOMICAL STRUCTURE AND PHYSIOLOGICAL SIGNIFICANCE OF THE VV
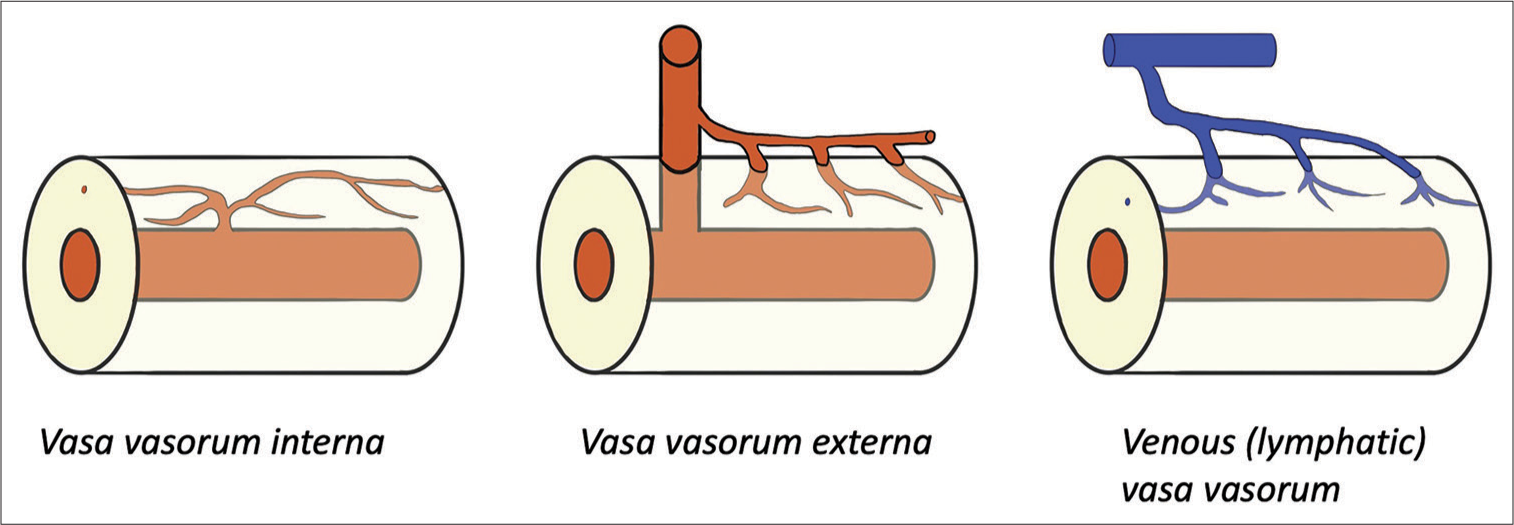
VVs are usually found on the adventitia of the parent vessel and infrequently reach the media and intima, depending on the size and type of the parent vessels and physiological and pathological conditions. Some articles referred to only VVs extending into the media as true VVs.[
The physiological function of VVs is to supply nutrition and oxygen to the parent vessel walls and to sustain their healthy physiological function, which has been well described in the previous literature. Geiringer investigated the VV distribution in the human aorta and coronary arteries and found that VVs are abundant in the adventitia and outer media. However, he also found that the inner layer of 0.5 mm or 0.35 mm in the aorta or coronary arteries from the lumen is always avascular. He defined this vascular-free layer as the “critical depth,” the limit of oxygen and nutrient diffusion from the lumen inside and outside VVs [
Wolinsky studied various mammalian VVs in the aorta and confirmed that critical depth can be seen in any species. The diffusion has been nicely visualized by Werber and Heistad[
INTRACRANIAL VV AND INTRACRANIAL VESSEL WALL NUTRITION
The intracranial vessels have been classically considered to lack VVs. Gimbert[
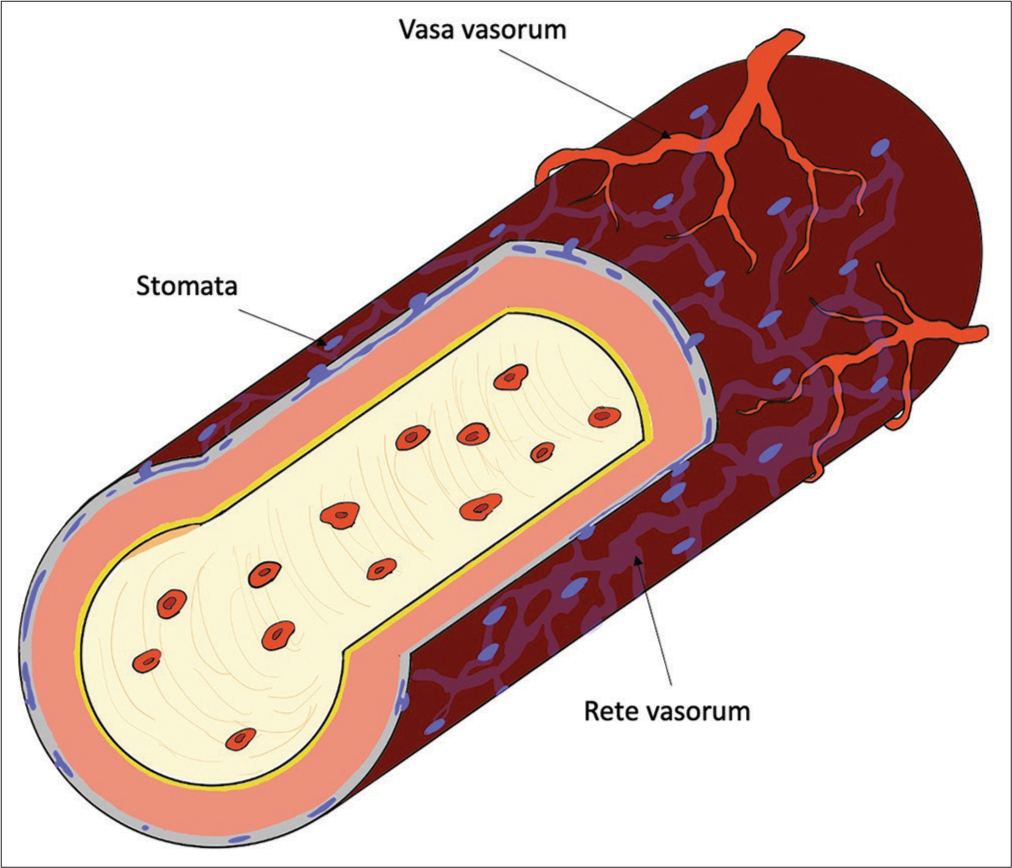
Figure 3:
Intracranial vessels and vasa vasorum (VV). In intracranial vessels, the VV is present only in the internal carotid and proximal vertebral arteries. The tunica adventitia of blood vessels in the subarachnoid space has tiny pores called stomata, and there is also a passageway for cerebrospinal fluid called the rete vasorum inside. Oxygen and nutrients are supplied to the tunica media through these channels. If a subarachnoid hemorrhage occurs and the vessel’s surface is covered with blood, this trophic pathway through the rete vasorum may cease functioning.
Pathological VV
Arteriosclerotic lesion
Atherosclerotic lesions are one of the most investigated pathologies involving VVs. In systemic atherosclerotic lesions, hypoxia and inflammation are the fundamental driving forces that trigger VV neovascularization. Atherosclerotic wall thickness directly obstructs diffusion and causes wall hypoxia.[
Induced VV hyperplasia, in turn, leads to the progression of atherosclerosis in many ways. Nakashima and Tashiro investigated lipoprotein deposition at the early stage of atherosclerotic lesions and found that the deposit was seen at the border zone between the intima and media rather than the innermost layer of the intima. The result suggested that the lipoprotein is delivered primarily by the VVs instead of the inner luminal flow.[
The involvement of VVs with intracranial atherosclerotic lesions has been described in only a limited number of articles. The intracranial vessels, as aforementioned, do not have VVs except for their proximal segments. However, VVs can be distally observed in intracranial atherosclerosis[
THROMBUS RESOLUTION AND SECONDARY AVS FORMATION
The thrombus resolution process is analogous to that of granulation formation in wound formation in wound healing and undergoes inflammation and tissue organization.[
Interestingly, some authors reported secondary AVSs on the thrombosed deep venous wall after its resolution.[
The etiology of secondary AVSs may explain why secondary pial arteriovenous fistula is rarely reported.[
CEREBRAL ANEURYSM AND SUBARACHNOID HEMORRHAGE (SAH)
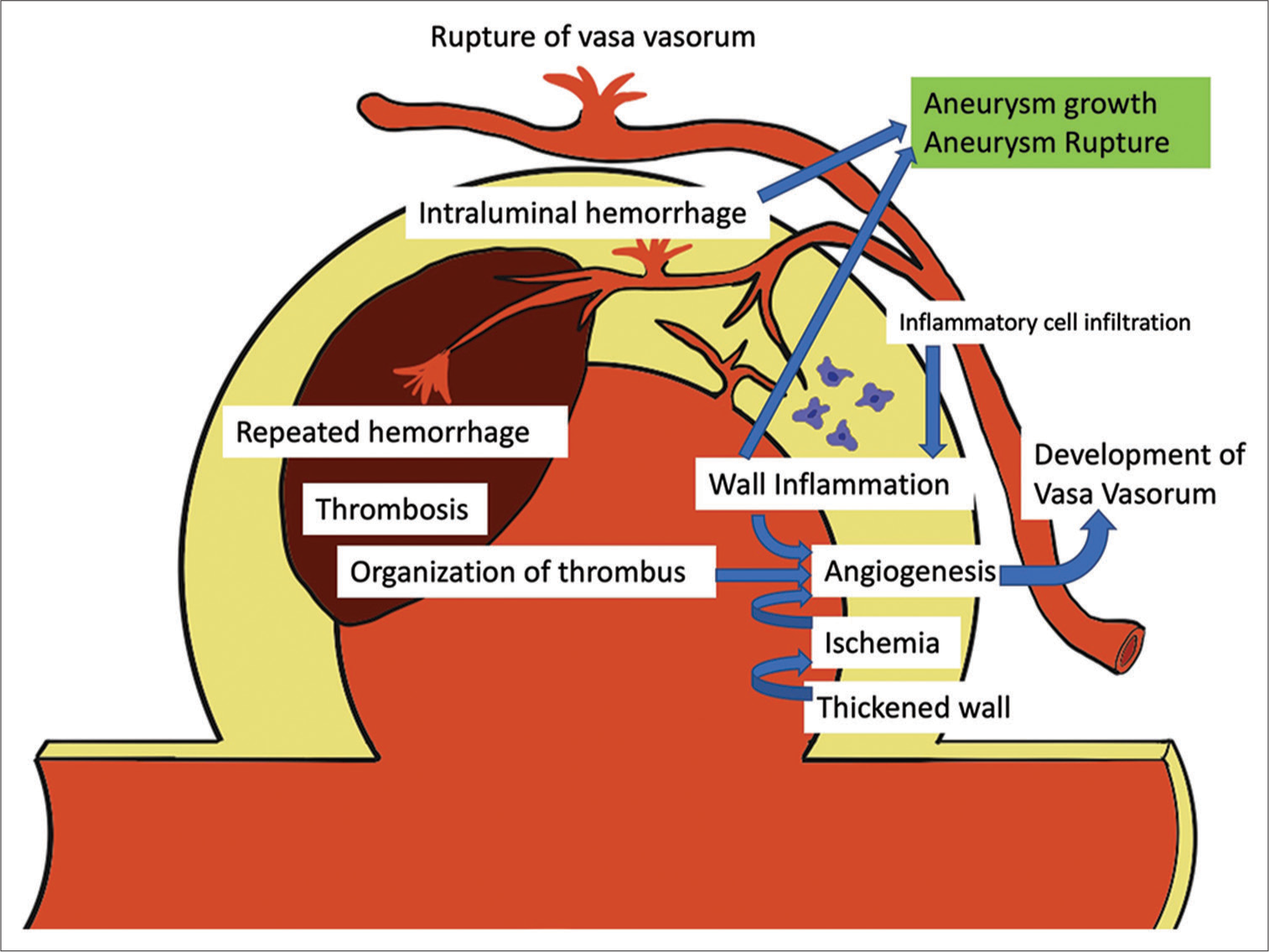
The association of VVs with unstable intracranial aneurysms inclined to grow or rupture has already been well recognized. As mentioned above, VVs are absent in intracranial vessels except in the proximal segment, but inflammation of the aneurysm wall, wall ischemia associated with decreased diffusion due to a thickened aneurysmal wall, and thrombosis in the aneurysm can trigger secondary VV angiogenesis in the aneurysm wall, as seen in the other pathologies. Induced VVs provide a route for inflammatory cell infiltration and accelerate aneurysm wall inflammation.[
The aneurysm rupture, SAH, affects the aforementioned peculiar way of nourishing the cerebral vasculature. Studies have reported that SAH induces severe subarachnoid vessel wall injury, including intimal edema and thickening, disruption of the internal elastic lamina, media thickening, smooth muscle cell necrosis, intramural hematoma, and VV recruitment in human cadavers[
Vessel wall imaging (VWI) using enhanced MRI is a modern imaging technique that can detect VV proliferation. In 1991, Atkinson first reported an association between aneurysmal wall enhancement and VVs on the aneurysmal wall.[
The recent advance in endovascular treatment of aneurysms further signifies the association between VVs and aneurysms. Both clipping surgery and endovascular coil embolization block blood influx into the aneurysm. However, they have a fundamental difference as a treatment concerning VVs; in clipping surgery, VVs are also clipped on the aneurysmal neck, while VVs externa remain intact. Accordingly, VVs still supply blood flow to the aneurysmal wall after coil embolization and deliver inflammatory cells, resulting in bleeding from VVs and aneurysmal growth. Indeed, some authors reported a case of a growing aneurysm even after angiographical complete occlusion had been achieved.[
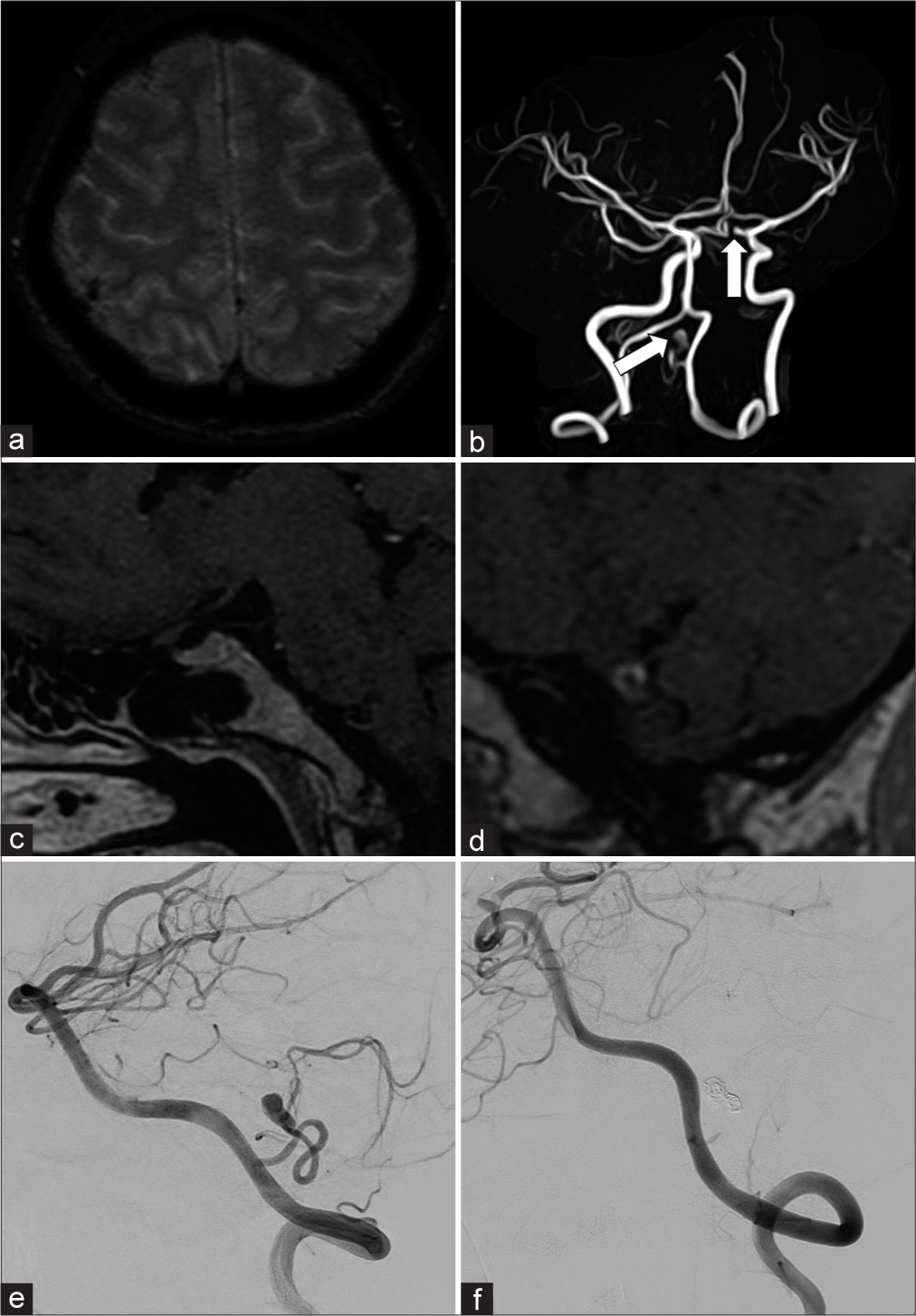
Figure 5:
Representative case of vessel wall imaging (VWI) in multiple aneurysms. A 47-year-old female visited our hospital 1 week after headache onset. (a) The initial magnetic resonance (MR) scan revealed cortical subarachnoid hemorrhage. (b) Subsequent MR angiography demonstrated two aneurysms (white arrows): The left internal carotid artery (ICA) aneurysm and the left posterior inferior cerebellar artery (PICA) aneurysm. (c and d) The VWI (enhanced MR) confirmed that the ruptured aneurysm was the left internal PICA aneurysm, and there was no enhancement in the left ICA aneurysm (c shows the left ICA aneurysm, d shows the left PICA aneurysm). (e and f) The PICA aneurysm was subsequently embolized with coils and glue (e: preinterventional radiology [IVR] digital subtraction angiography [DSA], f: post-IVR DSA).
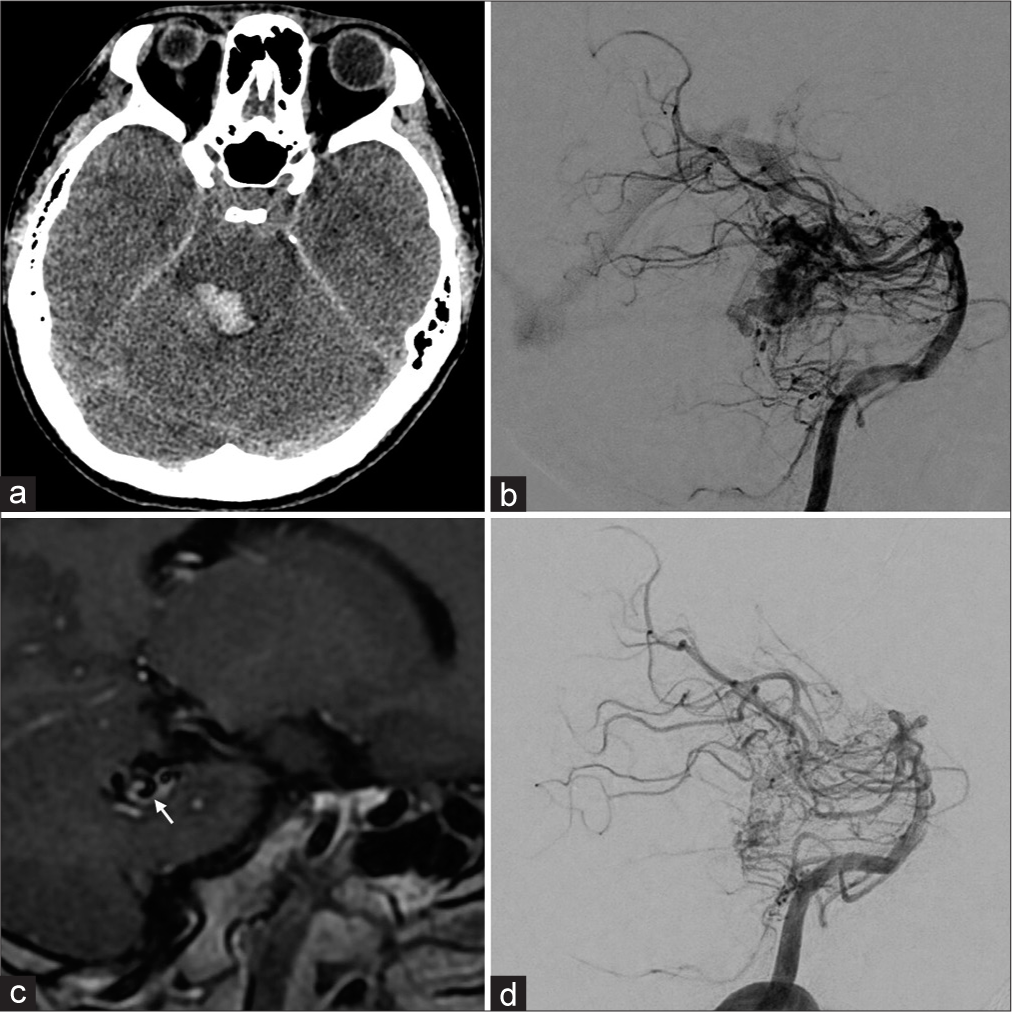
Figure 6:
Representative case of vessel wall imaging (VWI) in arteriovenous malformation (AVM). A 27-year-old male visited our hospital with headaches, diplopia, and sensory disturbance. (a) A computed tomography scan demonstrated brainstem hemorrhage. (b) Subsequent digital subtraction angiography (DSA) revealed a brain AVM in the dorsal aspect of the brain stem. (c) In addition, VWI showed vessel wall enhancement in the drainage vein (white arrow). (d) Postinterventional DSA showed a significant decrease in blood flow. Accordingly, reducing the flow burden was a feasible therapeutic option. The patient underwent two transarterial embolization sessions and further stereotactic radiosurgery for the remaining AVM.
COLLATERAL CHANNELS
On the surface of the vessels, VVs form an abundant network, and the VVs in different territories can anastomose with each other.[
A FUTURE TREATMENT TARGETING VVS
This article reviews past and recent articles and discusses the involvement of VVs in various intracranial lesions. In most lesions, VVs aggravate vascular wall inflammation and cause bleeding from or within the vascular wall or plaque, leading to pathological progression. Therefore, the involvement of VVs can be considered as a malignant lesion in one way. At present, no study specifically targeting VVs in intracranial lesions has been reported. However, in the future treatment of intracranial lesions, we need to consider VV involvement or target the VVs themselves. However, at the same time, VVs sustain the normal healthy vascular wall. Thus, complete obliteration of VVs may increase the risk of wall ischemia and medial necrosis. In our opinion, “leaky” pathological VVs should be targeted, and transforming pathological VVs into healthy physiological VVs may change the situation. Further research is needed to elucidate the involvement of VVs in intracranial lesions and facilitate better clinical outcomes.
CONCLUSION
VV is not only involved in the nutrition and metabolism of the vascular wall but is also deeply involved in the pathogenesis of inflammation, ischemia, and thrombosis of the vascular wall. In addition, in the central nervous system, intracranial vascular wall nutrient particularities and VVs are closely related to the pathogenesis of cerebral aneurysms, SAH, AVS disease, atherosclerotic lesions, and other conditions. We believe that the issues discussed in this paper are of great importance to neurosurgeons and endovascular surgeons.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Andres KH. On the fine structure of the arachnoidea and dura mater of mammals. Z Zellforsch Mikrosk Anat. 1967. 79: 272-95
2. Atkinson JL, Okazaki H, Sundt TM, Nichols DA, Rufenacht DA. Intracranial cerebrovascular vasa vasorum associated with atherosclerosis and large thick-walled aneurysms. Surg Neurol. 1991. 36: 365-9
3. Aydin F. Do human intracranial arteries lack vasa vasorum? A comparative immunohistochemical study of intracranial and systemic arteries. Acta Neuropathol. 1998. 96: 22-8
4. Berenstein A, Lasjaunias P, Brugge KG, editors. Clinical and endovascular treatment aspects in adults. Surgical neuroangiography. New York: Springer Science and Business Media; 2012. 2:
5. Björnheden T, Bondjers G. Oxygen consumption in aortic tissue from rabbits with diet-induced atherosclerosis. Arteriosclerosis. 1987. 7: 238-47
6. Björnheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol. 1999. 19: 870-6
7. Brandão GM, Sobreira ML, Malgor RD, Rollo HA. Recanalization rates after acute deep vein thrombosis: A single-center experience using a newly proposed vein diameter variation index. Ann Vasc Surg. 2014. 28: 1751-60
8. Clarke JA. An X-ray microscopic study of the vasa vasorum of the intracranial arteries. Z Anat Entwicklungsgesch. 1965. 124: 396-400
9. Clarke JA. An X-ray microscopic study of the vasa vasorum of the normal human ascending aorta. Heart. 1965. 27: 99-104
10. Clower BR, Sullivan DM, Smith RR. Intracranial vessels lack vasa vasorum. J Neurosurg. 1984. 61: 44-8
11. Colon GP, Deveikis JP, Dickinson LD. Revascularization of occluded internal carotid arteries by hypertrophied vasa vasorum: Report of four cases. Neurosurgery. 1999. 45: 634-7
12. Cord BJ, Renedo D, Santarosa C, Sujijantarat N, Antonios J, Kim JA. Vessel wall MRI in ruptured cranial dural arteriovenous fistulas. Interv Neuroradiol. 2021. 27: 553-7
13. Corti A, De Paolis A, Tarbell J, Cardoso L. Stenting-induced vasa vasorum compression and subsequent flow resistance: A finite element study. Biomech Model Mechanobiol. 2021. 20: 121-33
14. Drozdz K, Janczak D, Dziegiel P, Podhorska M, Patrzałek D, Ziółkowski P. Adventitial lymphatics of internal carotid artery in healthy and atherosclerotic vessels. Folia Histochem Cytobiol. 2008. 46: 433-6
15. Drozdz K, Janczak D, Dziegiel P, Podhorska M, Piotrowska A, Patrzalek D. Adventitial lymphatics and atherosclerosis. Lymphology. 2012. 45: 26-33
16. Edjlali M, Gentric JC, Régent-Rodriguez C, Trystram D, Hassen WB, Lion S. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms?. Stroke. 2014. 45: 3704-6
17. Eisenmenger LB, Junn JC, Cooke D, Hetts S, Zhu C, Johnson KM. Presence of vessel wall hyperintensity in unruptured arteriovenous malformations on vessel wall magnetic resonance imaging: Pilot study of AVM vessel wall “enhancement. ” Front Neurosci. 2021. 15: 697432
18. Espinosa F, Weir B, Shnitka T. Electron microscopy of simian cerebral arteries after subarachnoid hemorrhage and after the injection of horseradish peroxidase. Neurosurgery. 1986. 19: 935-45
19. Federspiel JM, Tschernig T, Laschke MW, Wagenpfeil S, Schnabel P, Schäfers HJ. The vasa vasorum reach deep into the human thoracic aorta. Ann Anat. 2019. 225: 54-6
20. Feng Y, Dmytriw AA, Yang B, Jiao L. Neovascularization in human intracranial atherosclerotic in-stent restenosis. Diagnostics (Basel). 2021. 11: 322
21. Gimbe. Memorie sur la structure et sur la texture des artères. J Anatom Physiol Norm Pathol Homme Anim. 1865. 2: 536-68
22. Gössl M, Rosol M, Malyar NM, Fitzpatrick LA, Beighley PE, Zamir M. Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec A Discov Mol Cell Evol Biol. 2003. 272: 526-37
23. Harnoss JM, Krackhardt F, Ritter Z, Granzow S, Felsenberg D, Neumann K. Porcine arteriogenesis based on vasa vasorum in a novel semi-acute occlusion model using high-resolution imaging. Heart Vessels. 2017. 32: 1400-9
24. Hassler O, Larsson SE. The external elastic layer of the cerebral arteries in different age-groups. Acta Anat (Basel). 1962. 48: 1-6
25. Hawkes C, Durant C, van Adel B. Symptomatic internal carotid artery vasa vasorum treated with surgical occlusion. Can J Neurol Sci. 2022. 49: 118-9
26. Heistad DD, Armstrong ML, Amundsen S. Blood flow through vasa vasorum in arteries and veins: effects of luminal PO2. Am J Physiol. 1986. 250: H434-42
27. His W, editors. Die anatomische nomenclatur. Nomina anatomica. Leipzig: Veit; 1895. p.
28. Hou Y, Ryu CH, Jun JA, Kim SM, Jeong CH, Jeun SS. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int. 2014. 38: 1050-9
29. Jurrus ER, Weiss HS. In vitro tissue oxygen tensions in the rabbit aortic arch. Atherosclerosis. 1977. 28: 223-32
30. Kai H, Kuwahara F, Tokuda K, Shibata R, Kusaba K, Niiyama H. Coexistence of hypercholesterolemia and hypertension impairs adventitial vascularization. Hypertension. 2002. 39: 455-9
31. Korkmaz E, Kleinloog R, Verweij BH, Allijn IE, Hekking LH, Regli L. Comparative ultrastructural and stereological analyses of unruptured and ruptured saccular intracranial aneurysms. J Neuropathol Exp Neurol. 2017. 76: 908-16
32. Krings T, Piske RL, Lasjaunias PL. Intracranial arterial aneurysm vasculopathies: Targeting the outer vessel wall. Neuroradiology. 2005. 47: 931-7
33. Küker W, Gaertner S, Nagele T, Dopfer C, Schoning M, Fiehler J. Vessel wall contrast enhancement: A diagnostic sign of cerebral vasculitis. Cerebrovasc Dis. 2008. 26: 23-9
34. Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: Its origin and pathophysiological significance. Hum Pathol. 1995. 26: 450-6
35. Labropoulos N, Bhatti AF, Amaral S, Leon L, Borge M, Rodriguez H. Neovascularization in acute venous thrombosis. J Vasc Surg. 2005. 42: 515-8
36. Lametschwandtner A, Minnich B, Kachlik D, Setina M, Stingl J. Three-dimensional arrangement of the vasa vasorum in explanted segments of the aged human great saphenous vein: Scanning electron microscopy and three-dimensional morphometry of vascular corrosion casts. Anat Rec A Discov Mol Cell Evol Biol. 2004. 281: 1372-82
37. Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997. 87: 267-74
38. Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995. 66: 149-73
39. Liszczak TM, Black PM, Varsos VG, Zervas NT. The microcirculation of cerebral arteries: A morphologic and morphometric examination of the major canine cerebral arteries. Am J Anat. 1984. 170: 223-32
40. Lowenberg RI, Shumacker HB. Experimental studies in vascular repair; morphologic observations of normal vasa vasorum. Yale J Biol Med. 1948. 20: 395-401
41. Ludwig CG, editors. De arteriarum tunicis. Lipsiae: Ex Officina Langenhemiana; p. 1747
42. Matouk CC, Mandell DM, Günel M, Bulsara KR, Malhotra A, Hebert R. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms. Neurosurgery. 2013. 72: 492-6
43. Modarai B, Burnand KG, Humphries J, Waltham M, Smith A. The role of neovascularisation in the resolution of venous thrombus. Thromb Haemost. 2005. 93: 801-9
44. Nakashima T, Tashiro T. Early morphologic stage of human coronary atherosclerosis. Kurume Med J. 1968. 15: 235-42
45. Noh CY. Vasa vasorum in deep vein thrombus recanalization. J Vasc Ultrasound. 2018. 42: 33-5
46. Numagami Y, Ezura M, Takahashi A, Yoshimoto T. Antegrade recanalization of completely embolized internal carotid artery after treatment of a giant intracavernous aneurysm: A case report. Surg Neurol. 1999. 52: 611-6
47. O’Nejll JF. The effects on venous endothelium of alterations in blood flow through the vessels in vein walls, and the possible relation to thrombosis. Ann Surg. 1947. 126: 270-88
48. Omodaka S, Endo H, Fujimura M, Niizuma K, Sato K, Matsumoto Y. High-grade cerebral arteriovenous malformation treated with targeted embolization of a ruptured site: Wall enhancement of an intranidal aneurysm as a sign of ruptured site. Neurol Med Chir. 2015. 55: 813-7
49. Ozoner B, Cakir T, Kayaci S, Aydin MD, Aydin S, Demirci E. Effect of vasa vasorum on basilar artery vasospasm following subarachnoid hemorrhage. World Neurosurg. 2019. 131: e218-25
50. Petridis AK, Dibue-Adjei M, Cornelius JF, Suresh MP, Li L, Kamp MA. Contrast enhancement of vascular walls of intracranial high flow malformations in black blood MRI indicates high inflammatory activity. Chin Neurosurg J. 2018. 4: 13
51. Phatouros CC, Halbach VV, Dowd CF, Lempert TE, Malek AM, Meyers PM. Acquired pial arteriovenous fistula following cerebral vein thrombosis. Stroke. 1999. 30: 2487-90
52. Phillippi JA. On vasa vasorum: A history of advances in understanding the vessels of vessels. Sci Adv. 2022. 8: eabl6364
53. Quan K, Song J, Zhu W, Wang D, An Q, Huang L. Validation of wall enhancement as a new imaging biomarker of unruptured cerebral aneurysm. Stroke. 2019. 50: 1570-3
54. Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009. 53: 1517-27
55. Standring S, editors. Gray’s anatomy: The anatomical basis of clinical practice. Netherlands: Elsevier; 2020. p.
56. Takaba M, Endo S, Kurimoto M, Kuwayama N, Nishijima M, Takaku A. Vasa vasorum of the intracranial arteries. Acta Neurochir (Wien). 1998. 140: 411-6
57. Terada T, Higashida RT, Halbach VV, Dowd CF, Tsuura M, Komai N. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994. 80: 884-9
58. Texakalidis P, Hilditch CA, Lehman V, Lanzino G, Pereira VM, Brinjikji W. Vessel wall imaging of intracranial aneurysms: Systematic review and meta-analysis. World Neurosurg. 2018. 117: 453-8.e1
59. Tsang CP, Mizutani K, Trenkler J, Holmin S, Rodesch G. De novo arteriovenous shunts after endovascular cure of cerebrospinal macro arteriovenous fistulas. A role for the vasa vasorum?. J Neuroradiol. 2021. 48: 127-31
60. Wakefield TW, Linn MJ, Henke PK, Kadell AM, Wilke CA, Wrobleski SK. Neovascularization during venous thrombosis organization: A preliminary study. J Vasc Surg. 1999. 30: 885-92
61. Wang R, Weng L, Li M. Effect of vasa vasorum in cerebrovascular compensation: 2 case reports. Ann Transl Med. 2020. 8: 508
62. Werber AH, Heistad DD. Diffusional support of arteries. Am J Physiol. 1985. 248: H901-6
63. Wilens SL, Malcolm JA, Vazquez JM. Experimental infarction (medial necrosis) of the dog’s aorta. Am J Pathol. 1965. 47: 695-711
64. Williams JK, Armstrong ML, Heistad DD. Vasa vasorum in atherosclerotic coronary arteries: Responses to vasoactive stimuli and regression of atherosclerosis. Circ Res. 1988. 62: 515-23
65. Willis T, editors. Pharmaceutice rationalis, sive, diatriba de medicamentorum operationibus in humano corpore. Oxford: E Theatro Sheldoniano; p. 1674
66. Xu J, Lu X, Shi GP. Vasa vasorum in atherosclerosis and clinical significance. Int J Mol Sci. 2015. 16: 11574-608
67. Yamashita A, Shoji K, Tsuruda T, Furukoji E, Takahashi M, Nishihira K. Medial and adventitial macrophages are associated with expansive atherosclerotic remodeling in rabbit femoral artery. Histol Histopathol. 2008. 23: 127-36
68. Yuan H, Sun J, Zhou Z, Qi H, Wang M, Dong D. Diagnosis and treatment of acquired arteriovenous fistula after lower extremity deep vein thrombosis. Int Angiol. 2019. 38: 10-6
69. Zervas NT, Liszczak TM, Mayberg MR, Black PM. Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. J Neurosurg. 1982. 56: 475-81
70. Zhang M, Cresswell N, Tavora F, Mont E, Zhao Z, Burke A. Instent restenosis is associated with neointimal angiogenesis and macrophage infiltrates. Pathol Res Pract. 2014. 210: 1026-30
71. Zhong W, Su W, Li T, Tan X, Chen C, Wang Q. Aneurysm wall enhancement in unruptured intracranial aneurysms: A histopathological evaluation. J Am Heart Assoc. 2021. 10: 1026-30