- Department of Neurosurgery, Hannover Medical School, Hannover, Germany
- Department of Neurosurgery, Klinikum Dessau, Dessau-Rosslau, Germany,
- House Officer, Faculty of Medicine, Assiut University, Assiut, Egypt,
- Department of Medicine, University of Kufa, Najaf, Iraq,
- Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon,
- Department of Neurosurgery, Sapienza University of Rome, Rome, Italy,
- Faculty of Medicine, Mohammed V University, Rabat, Morocco,
- Department of Psychiatry, University of Birmingham, Birmingham, United Kingdom,
- Department of Neurosurgery, Institute of Medical Sciences and SUM Hospital, Bhubaneswar, Odisha, India
- 0Department of Neurosurgery, Trivandrum Medical College, Trivandrum, Kerala, India,
- 1Department of Neurosurgery, Imperial College Healthcare National Health Services Trust, Charing Cross Hospital, London, United Kingdom.
Correspondence Address:
Oday Atallah, Department of Neurosurgery, Hannover Medical School, Hannover, Germany.
DOI:10.25259/SNI_215_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Oday Atallah1, Amr Badary2, Fatma A. Monib3, Yasser F. Almealawy4, Aalaa Saleh5, Francesco Lioi6, Souhaila Fathallah7, Apil Sapkota8, Mrinmoy Kundu9, Vivek Sanker10, Joe M. Das11. Ventriculoperitoneal shunt extrusion in pediatric patients, clinical patterns and therapeutic strategies: A scoping review. 05-Jul-2024;15:226
How to cite this URL: Oday Atallah1, Amr Badary2, Fatma A. Monib3, Yasser F. Almealawy4, Aalaa Saleh5, Francesco Lioi6, Souhaila Fathallah7, Apil Sapkota8, Mrinmoy Kundu9, Vivek Sanker10, Joe M. Das11. Ventriculoperitoneal shunt extrusion in pediatric patients, clinical patterns and therapeutic strategies: A scoping review. 05-Jul-2024;15:226. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12982
Abstract
Background: Ventriculoperitoneal shunts (VPSs) are frequently employed in neurosurgery to treat hydrocephalus, with a particular focus on pediatric patients. Although VPSs are commonly utilized, they are not exempt from difficulties, such as shunt extrusion. The main aim of this study is to enhance comprehension regarding the occurrence, causes contributing to, and consequences of VPS extrusion in pediatric patients.
Methods: A comprehensive search approach was implemented, including electronic databases, including PubMed, Google Scholar, and Scopus, to locate pertinent articles published between January 1950 and May 2023. The utilization of keywords such as “ventriculoperitoneal shunt” and “extrusion,” “ventriculoperitoneal shunt” and “migration,” and “ventriculoperitoneal shunt” and “perforation” was employed. Data on patient demographics, underlying diseases, origin of extrusion, presenting symptoms, treatment, and follow-up were gathered. Statistical studies were conducted to identify potential risk factors connected with the occurrence of shunt extrusion.
Results: A study analyzed 80 studies on 120 individuals with extruded VPS catheters. The majority of patients (55.8%) had symptoms such as cerebrospinal fluid leakage and irritation. Hydrocephalus was categorized into congenital (40%), obstructive (36.7%), and communicating (11.7%) groups. Catheter extrusion sites varied, with most from the anal or rectal site. Preoperative meningitis or peritonitis was present in 20% of patients. Treatments ranged from shunt removal to endoscopic third ventriculostomy, resulting in a 90% recovery rate, 1.7% mortality, and 5% follow-up loss.
Conclusion: Extrusion of the distal catheter in VPSs is a critical medical situation that necessitates urgent surgical intervention. The presence of an infection raises the likelihood of complications; hence, it is vital to promptly address the issue through the administration of antibiotics and the replacement of the shunt. Timely intervention enhances results.
Keywords: Child, Children, Extrusion, Hydrocephalus, Neurosurgery, Pediatric, Perforation, Ventriculoperitoneal shunt
INTRODUCTION
Ventriculoperitoneal shunts (VPSs) have evolved into a crucial and widely used neurosurgical intervention for managing hydrocephalus, a neurological disorder characterized by an abnormal cerebrospinal fluid (CSF) accumulation within the cerebral ventricles.[
The primary function of these components is to allow excess CSF to drain away from the cranial vault, reducing intracranial pressure (ICP) and alleviating associated neurological symptoms. This intricate system relies on pressure differentials to allow CSF to flow from the cerebral ventricles, through the proximal catheter, along the subcutaneous path, and finally into the peritoneal cavity, where the body’s natural mechanisms reabsorb it. The regulation of CSF flow is further facilitated by a valve mechanism, often located along the distal catheter or within the shunt’s programmable unit, which can be adjusted to optimize CSF diversion based on individual patient needs.[
Although VPSs have significantly transformed the management of hydrocephalus, it is crucial to acknowledge that intricacies and potential consequences accompany their utilization.[
This review aims to provide a comprehensive analysis of shunt extrusion, encompassing its occurrence, underlying pathophysiological causes, clinical symptoms, diagnostic techniques, and the range of therapeutic options currently utilized.
MATERIALS AND METHODS
The aims and objectives of the study were carefully considered, and a protocol was written to reflect that. Methods for conducting the search, extracting data, synthesizing data, determining which should be included, evaluating the quality of the studies, and ultimately screening them were all documented in the protocol. This review follows the guidelines established by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
All of the studies reported in the literature in English with participants younger than 18 years were considered for inclusion. Patients older than 18 years old, studies that reported only the migration of the VPS without extrusion, studies that provided an overview, and studies conducted in languages other than English were not considered.
We identified a database of studies on the extrusion of VPSs in the pediatric population through various orifices by conducting a systematic review and searching the relevant literature using PubMed, Google Scholar, and Scopus. The search terms “ventriculoperitoneal shunt” and “extrusion,” “ventriculoperitoneal shunt” and “migration,” and “ventriculoperitoneal shunt” and “perforation” were employed.
To find the articles that met the systematic review’s inclusion criteria, we screened their titles and abstracts and then read them in their entirety. The studies that met the inclusion and exclusion criteria were subjected to a thorough critical review by two reviewers, O.A. and Y.A. We first looked at the titles with keywords, then at the abstracts, and finally at the full texts of those that seemed relevant. At the full-text screening stage, all studies were reviewed by an independent reviewer, A.B., to ensure eligibility. Articles selected or included differently by each of the three reviewers were briefly discussed until a consensus was reached [
The third review resolved any discrepancies that emerged following critical analysis, while the first two reviews independently determined the risk of bias and carried out data extraction using a designed protocol by the Cochrane Handbook.[
Statistical analysis was carried out using Microsoft Excel and the statistical software package Statistical Package for the Social Sciences 26. Categorical data were compared and tested for significance using the Chi-square test. Statistical significance was defined as P < 0.05.
RESULTS
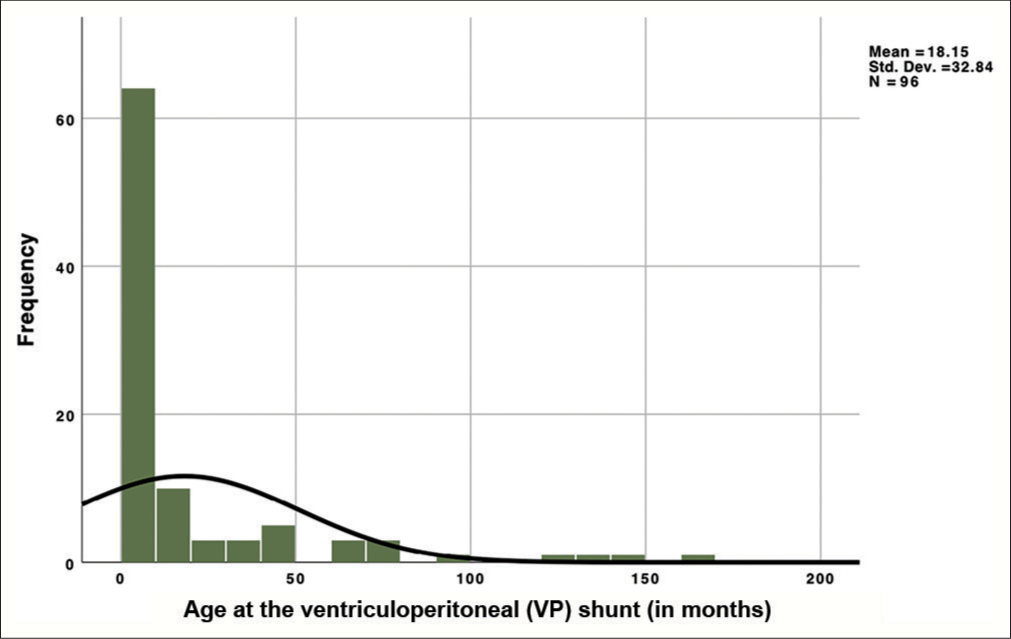
Following a comprehensive screening process, a selection of 80 studies was undertaken, employing rigorous criteria during the initial search across reputable databases, including PubMed, Scopus, and Google Scholar. This extensive study analyzed patient-level data derived from a defined cohort comprising 120 individuals, all grappling with the challenging clinical scenario of the extruded distal end of the VPS catheter. Our age distribution revealed that patients within our study were administered their first shunt at a median age of 3 months, while 75% of participants were below 18 months [
Gender analysis demonstrated that out of these individuals, 64 were unequivocally identified as male, constituting 53.3% of the total cohort. In contrast, 48 were unequivocally identified as females, accounting for 40% of the cohort, while the genders of the remaining 8 patients (6.7%) were unknown.
Remarkably, the gender of the remaining participants remained enigmatic, and our analysis disclosed no statistically significant gender preferences. It is essential to underscore that a substantial proportion of our cohort, precisely 67 patients, making up a significant 55.8%, presented with a spectrum of symptoms. These symptoms manifested diversely and were likely attributable to localized effects, including the leakage of CSF and irritation of the mucosal lining of the affected organ due to the infected distal catheter. These localized effects often manifest as distressing symptoms such as vomiting, skin erosion, and purulent discharge. Conversely, some of the reported symptoms may have originated from systemic issues linked to VPS dysfunction or infection, resulting in altered mental status, fever, and sepsis. A comprehensive and thoughtful compilation of imaging findings for each individual was organized [
The classification of hydrocephalus represents a pivotal facet of our study, and we categorized it into three principal groups for our statistical analysis: congenital (n = 48, 40%), obstructive (n = 44, 36.7%), and communicating (n = 14, 11.7%), while the type in the rest 14 patients (11.7%) was unknown. This deliberate categorization provides valuable insights into our study’s broad spectrum of conditions.
The origin of catheter extrusion emerged as a significant aspect of our analysis. Our findings strongly suggest that in 44 patients (36.7%) of the cases, the catheter extruded from the anal or rectal site. Conversely, the remaining 17 patients (14.2%) experienced extrusion from the oral site, 14 patients (11.7%) extruded from the abdominal wall, 9 patients (7.5%) from the vagina, 8 patients (6.7%) from the urethra, 7 patients (5.8%) from the umbilicus, and 6 patients (5%) from an inguinal site. The remaining 12 patients (12.5%) exhibited extrusion from random sites [
An important variable in our study is the presence of preoperative meningitis or peritonitis in only 24 patients (20%). Our analysis found no statistically significant difference in peritonitis or meningitis presentation between patients presenting across the three primary extrusion sites (oral or rectal, abdominal wall, and urethra), with P = 0.743.
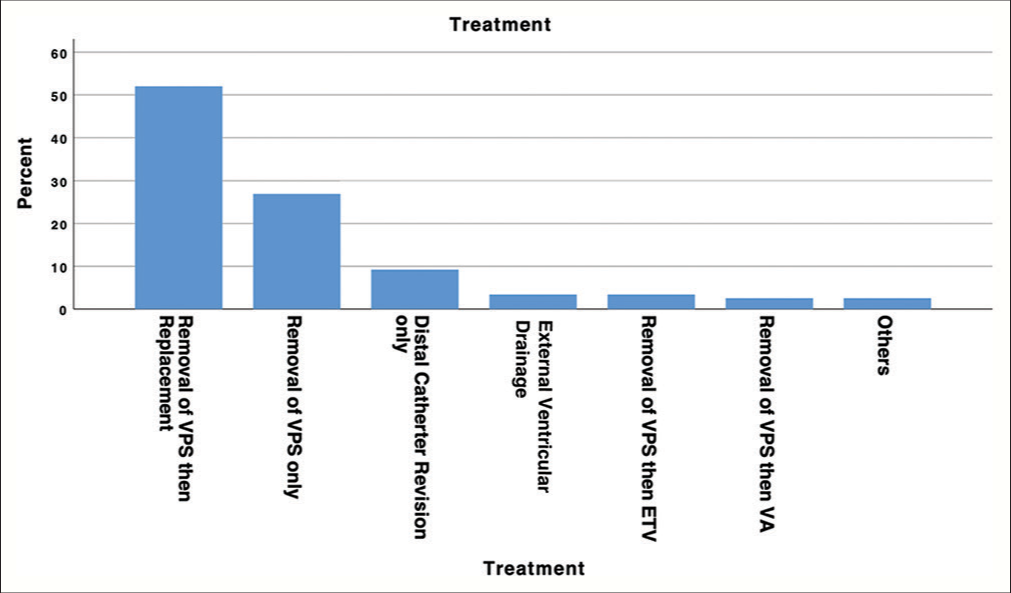
Irrespective of the presenting symptoms, all patients underwent invasive management procedures. The spectrum of treatments employed was diverse and included various approaches: 62 patients (51.7%) underwent the removal of the VPS with subsequent replacement during the same hospital admission; 32 patients (26.7%) had the VPS removed exclusively; 11 patients (9.2%) underwent distal catheter revision alone; 4 patients (3.3%) underwent VPS removal followed by endoscopic third ventriculostomy (ETV); 3 patients (2.5%) underwent external ventricular drainage (EVD) without mentioning the further management; and 3 patients (2.5%) had the VPS removed and replaced with a ventriculoatrial (VA) shunt. The remaining 3 patients (2.5%) underwent alternative treatment management strategies. In addition, there was one patient for whom the therapy remained enigmatic [
DISCUSSION
The distal catheter of the VPS typically resides serenely within the peritoneal cavity, navigating its course amidst the labyrinthine folds of the intestines without causing any disruption. However, under certain predisposing conditions, it embarks on a transformative odyssey, migrating to atypical locations where it adheres and initiates an inflammatory cascade. Abdominal wall contractions can exert force on the catheter, propelling it into the encircling fibrous tract. This phenomenon may follow episodes of elevated intra-abdominal pressure or may result from anchoring to a calcified point along the catheter’s path, thereby prompting its migration toward subcutaneous tissues.[
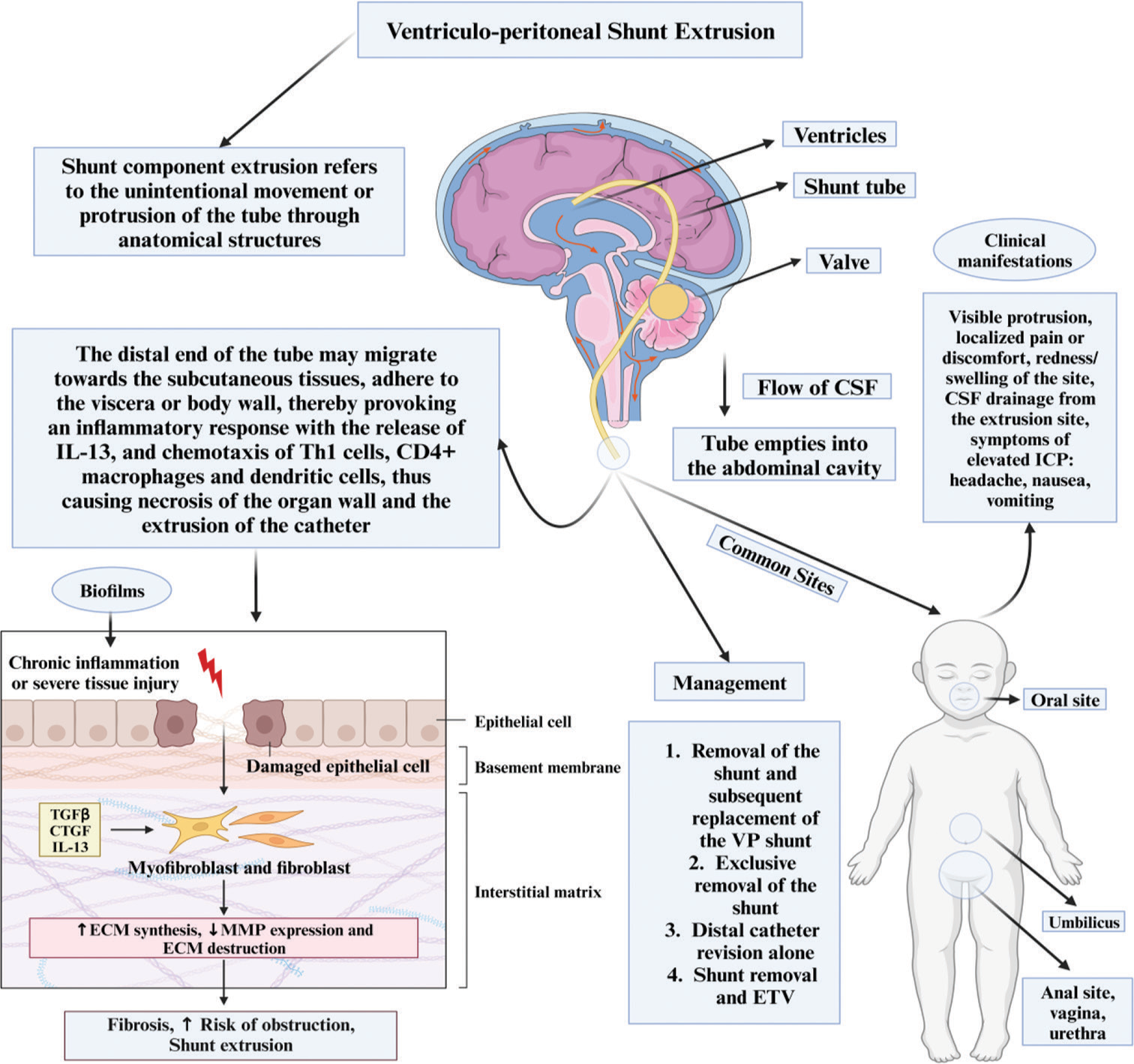
Figure 5:
Overview of the ventriculoperitoneal shunt extrusion. IL-13: Interleukin 13, TGF: Transforming Growth Factor, CTGF: Connective Tissue Growth Factor, ECM: Extracellular Matrix, MMP: Matrix Metalloproteinease, ICP: Intracranial Pressure, ETV: External Third Ventriculostomy, VP Shunt: Ventriculoperitoneal shunt
This journey through uncharted territories triggers intricate foreign body tissue responses – inflammatory cells, collagen deposition, and fibrous tissue encapsulation – that are intricately linked to the risk of catheter extrusion, ultimately culminating in one of the most prevalent complications – obstruction, a frequent impetus for revision surgeries.[
When delving into the realm of other potential predisposing factors, infection emerges as a prominent contender when localized around the shunt components. This infectious component holds the potential to exert a considerable influence on the likelihood of shunt extrusion. This revelation harmonizes seamlessly with the prevailing body of neurosurgical literature, which underscores the pivotal role of infection in the landscape of shunt-related complications.[
The clinical presentation of distal catheter extrusion in a VPS can vary depending on the extent of catheter displacement and associated complications. Common clinical signs and symptoms associated with distal catheter extrusion may include visible protrusion of the catheter from the abdomen, usually at the site where it was initially placed;[
The extrusion site can become a potential entry point for infection, leading to symptoms such as fever, increased pain, or redness.[
When it comes to the management of extrusion of the distal catheter from a VPS through the abdominal wall, the prompt administration of prophylactic antibiotics is essential, followed by complete replacement of the entire shunt system.[
Some studies investigated the outcomes of pediatric patients who underwent VPS revision for various complications, including extrusion, and found that early intervention and meticulous surgical techniques were associated with improved outcomes and reduced recurrence rates.[
In recurring infections and complications associated with VPSs, a potential solution may involve transitioning to a VA shunt. This approach, as reported in some studies, has been linked to enhanced clinical outcomes and a reduction in ventricular size,[
CONCLUSION
Distal catheter extrusion in VPSs involves the unintended protrusion of the catheter from its intended exit site, causing clinical symptoms such as visible protrusion, localized pain, redness, swelling, CSF drainage, and signs of infection. Prompt management is crucial, typically involving prophylactic antibiotics and complete shunt system replacement, with the timing of reinsertion determined by CSF analysis results, indicating feasibility for immediate reinsertion.
Extrusion sites vary, including the anal or rectal area, abdominal wall, urethra, vagina, umbilicus, and inguinal region, each presenting unique management challenges. Distal catheter extrusion is a medical emergency, necessitating immediate medical attention, surgical intervention, and shunt revision to prevent complications such as infection or intracranial hypertension. Alternative drainage options, such as VASs and ETV with postoperative EVD, may be considered based on the clinical scenario and patient characteristics. Managing distal catheter extrusion has diverse outcomes, with early intervention, meticulous surgical techniques, and appropriate strategies associated with improved outcomes and reduced recurrence rates. Continued research is essential to refine treatment approaches for the varied distribution of extrusion sites in VPSs.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agarwal M, Adhana R, Namdev H, Yadav YR, Agrawal T. Transoral extrusion of the ventriculo-peritoneal shunt: A case report and review of literature. J Pediatr Neurosci. 2011. 6: 149-51
2. Agarwal N, Shukla RM, Agarwal D, Gupta K, Luthra R, Gupta J. Pediatric ventriculoperitoneal shunts and their complications: An analysis. J Indian Assoc Pediatr Surg. 2017. 22: 155-7
3. Ahmadvand S, Dayyani M, Etemadrezaie H, Ghorbanpour A, Zarei R, Shahriyari A. Rate and risk factors of early ventriculoperitoneal shunt revision: A five-year retrospective analysis of a referral center. World Neurosurg. 2020. 134: e505-11
4. Akcora B, Serarslan Y, Sangun O. Bowel perforation and transanal protrusion of a ventriculoperitoneal shunt catheter. Pediatr Neurosurg. 2006. 42: 129-31
5. Al Fauzi A, Djatisoesanto W, Wahyuhadi J, Parenrengi MA, Turchan A. A rare case of repeated migration and transurethral extrusion of ventriculoperitoneal shunt. J Pediatr Neurosci. 2017. 12: 96-8
6. Alalula T, Alaqeel A, Almodhen F, Moneir W. Laparoscopic management of ventriculoperitoneal shunt extrusion through urethra in an infant: Case report and review of literature. Urol Case Rep. 2022. 43: 102099
7. Alfandy Nazwar T, Balafif F, Wardhana DW, Hanareta Hantoko S, Mustofa M. Transanal protrusion ventriculoperitoneal shunt migration in hydrocephalus patients. KMJ. 2023. 3: 52-4
8. Alhassan BA, Agyen-Mensah K, Rahman GA, Makafui CS. Ventriculoperitoneal shunt migration through the anus in a child: Case report and management algorithm. J Adv Med Med Res. 2020. 32: 53-7
9. Allouh MZ, Al Barbarawi MM, Hiasat MH, Abuzayed BA. Migration of the distal catheter of the ventriculoperitoneal shunt in hydrocephalus patients. Neurosciences (Riyadh). 2017. 22: 298-302
10. Alolyani A, Al Dandan F, Al-Umran S, Ammar A. Extrusion of anterior abdominal wall by a ventriculoperitoneal shunt-an uncommon complication: Case report and literature review. Asian J Neurosurg. 2020. 15: 425-7
11. Altas M, Tutanc M, Aras M, Altas ZG, Arica V, editors. Vaginal perforation caused by distal tip of ventriculoperitoneal shunt: Report of a rare complication. p.
12. Ansari S, Nejat F, Dadmehr M. Extrusion of ventriculoperitoneal shunt catheter through the rectum and retrograde meningitis. Pediatr Infect Dis J. 2005. 24: 1027
13. Aricó M, Beluffi G, Fiori P, Chiari G, Pezzotta S, Podesta AF. Rectal extrusion of the catheter and air ventriculography following bowel perforation in ventriculo-peritoneal shunt. Pediatr Radiol. 1985. 15: 53-5
14. Arnaout MM, Hoz SS, Bessar AA, Agrawal A, AbdulAzeez MM, Moscote-Salazar LR. Extrusion of a peritoneal catheter of a ventriculoperitoneal shunt from the Urethra. Neurol India. 2021. 69: 214-6
15. Ashpole R, Boulton R, Holmes AE. A case of asymptomatic passage per-rectum of a fractured redundant peritoneal catheter from a ventriculo-peritoneal shunt. Eur J Pediatr Surg. 1995. 5: 280-1
16. Badri M, Gader G, Belkahla G, Kallel J, Zammel I. Transoral migration of the inferior end of a ventriculoperitoneal shunt: A case report with literature review. Neurochirurgie. 2018. 64: 203-5
17. Bakhaidar M, Wilcox JT, Sinclair DS, Diaz RJ. Ventriculoatrial shunts: Review of technical aspects and complications. World Neurosurg. 2022. 158: 158-64
18. Bakshi S. Spontaneous trans-anal extrusion of caudally migrated ventriculo-peritoneal shunt tip in a child: A case report. Surg Case Rep. 2020. 6: 50
19. Bal’afif F, Wardhana DW, Nazwar TA, Nastiti NA. Anal extrusion of ventriculoperitoneal shunt: A case report and review of literature. J Kedokteran Brawijaya. 2021. 31: 269-72
20. Bansal H, Gupta G, Gupta M, Kaushal R. Unusual ventriculoperitoneal (VP) shunt tube extrusion through anus in a child with dandy walker malformation: A rare case report. J Clin Diagn Res. 2015. 9: D25-6
21. Bayston R, Brant C, Dombrowski SM, Hall G, Tuohy M, Procop G. An experimental in-vivo canine model for adult shunt infection. Cerebrospinal Fluid Res. 2008. 5: 17
22. Benachinmardi KK, Ravikumar R, Indiradevi B. Role of biofilm in cerebrospinal fluid shunt infections: A study at tertiary neurocare center from South India. J Neurosci Rural Pract. 2017. 8: 335-41
23. Berhouma M, Messerer M, Houissa S, Khaldi M. Transoral protrusion of a peritoneal catheter: A rare complication of ventriculoperitoneal shunt. Pediatr Neurosurg. 2008. 44: 169-71
24. Bodeliwala S, Agrawal A, Mittal A, Singh D, Vageesh BG, Singh H. Transanal protrusion of ventriculoperitoneal shunt via appendicular perforation: A rare case report. J Pediatr Neurosci. 2016. 11: 274-6
25. Borkar SA, Satyarthee GD, Khan RN, Sharma BS, Mahapatra AK. Spontaneous extrusion of migrated ventriculoperitoneal shunt catheter through chest wall: A case report. Turk Neurosurg. 2008. 18: 95-8
26. Bosy HH, Albarnawi BM, Ashour KM, Alyasi A, Alsulaihebi AS. Early anal protrusion of distal ventriculoperitoneal catheter due to iatrogenic colonic perforation: A case report and review of literature. Cureus. 2021. 13: e20296
27. Cardinale JP, Carrillo CO, Colón J. Perioperative management for rectal migration of a ventriculoperitoneal shunt. Ochsner J. 2020. 20: 239-41
28. Chiang LL, Kuo MF, Fan PC, Hsu WM. Transanal repair of colonic perforation due to ventriculoperitoneal shunt--case report and review of the literature. J Formos Med Assoc. 2010. 109: 472-5
29. Cho KR, Yeon JY, Shin HJ. Upward migration of a peritoneal catheter following ventriculoperitoneal shunt. J Korean Neurosurg Soc. 2013. 53: 383-5
30. Chugh A, Gotecha S, Amle G, Patil A, Punia P, Kotecha M. Abnormal migration and extrusion of abdominal end of ventriculoperitoneal shunt: An experience of eight cases. J Pediatr Neurosci. 2018. 13: 317-21
31. De Aguiar GB, Mizrahi C, Aquino JH, Tavares CM, Telles C, Nigri F. Urethral extrusion of a peritoneal catheter in a patient with a neobladder: A rare complication of shunt insertion. Neuropediatrics. 2011. 42: 124-7
32. De Jong L, Van Der Aa F, De Ridder D, Van Calenbergh F. Extrusion of a ventriculoperitoneal shunt catheter through an appendicovesicostomy. Br J Neurosurg. 2011. 25: 115-6
33. Digray NC, Thappa DR, Arora M, Mengi Y, Goswamy HL. Silent bowel perforation and transanal prolapse of a ventriculoperitoneal shunt. Pediatr Surg Int. 2000. 16: 94-5
34. Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998. 43: 294-303 discussion 303-305
35. Dua R, Jain R. Peroral extrusion of ventriculoperitoneal shunt: A case report and review of the literature. Cent Eur Neurosurg. 2011. 72: 107-8
36. Eser O, Dogru O, Aslan A, Kundak AA. Umbilical perforation: An unusual complication of a ventriculoperitoneal shunt. Childs Nerv Syst. 2006. 22: 1509-10
37. Fermin S, Fernández-Guerra RA, Sureda PJ. Extrusion of peritoneal catheter through the mouth. Childs Nerv Syst. 1996. 12: 553-5
38. Ferras M, McCauley N, Stead T, Ganti L, Desai B. Ventriculoperitoneal shunts in the emergency department: A review. Cureus. 2020. 12: e6857
39. Fowler JB, De Jesus O, Mesfin FB, editors. Ventriculoperitoneal shunt. StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. p.
40. Gan AM, Duke LD, Schwarz R, Power N. Extrusion of ventricular-peritoneal shunt through the urethra: Pediatric case report and literature review. Ann Clin Case Rep. 2017. 2: 1423
41. Garegnani L, Franco JV, Ciapponi A, Garrote V, Vietto V, Portillo Medina SA. Ventriculo-peritoneal shunting devices for hydrocephalus. Cochrane Database Syst Rev. 2020. 6: CD012726
42. Gatto LA, Mathias R, Tuma R, Abdalla R, De Aguiar PH. Rare complication of ventriculoperitoneal shunt: Catheter protrusion to subcutaneous tissue-case report. Surg Neurol Int. 2016. 7: S1142-6
43. Gelabert González M. Extrusion of peritoneal catheter through the anus. Childs Nerv Syst. 1987. 3: 183-4
44. Ghritlaharey RK. Ventriculoperitoneal shunt disconnection, shunt migration, and silent bowel perforation in a 10-year-old boy. J Neurosci Rural Pract. 2019. 10: 342-5
45. Griffith JA, DeFeo D. Peroral extrusion of a ventriculoperitoneal shunt catheter. Neurosurgery. 1987. 21: 259-61
46. Guimarães AS, Vaz Júnior M, Martins SP, Fagundes-Pereyra WJ. Rare case of migration and perforation of the urinary bladder by ventriculoperitoneal shunt catheter with intravesical knotted formation: A case report and literature review. Surg Neurol Int. 2022. 13: 75
47. Indra Gunawan P, Gunadi Ranuh IR, Fardah Atthiyah A. Anal extrusion of the ventriculoperitoneal shunt catheter. Acta Med Acad. 2017. 46: 65-6
48. Gupta M, Digra NC, Sharma N, Goyal S, Agrawal A. Peroral extrusion of the peritoneal catheter in an infant. N Am J Med Sci. 2012. 4: 290-2
49. Gupta R, Mala TA, Gupta A, Paul R, Malla SA, Gupta AK. Transoral migration of peritoneal end of ventriculoperitoneal shunt with perforation of gastro-esophageal junction: A case report of a rare complication. Bangladesh J Med Sci. 2014. 13: 492-5
50. Gupta SK, Jaiswal AK, Kumar S. Ventriculoperitoneal shunt catheter masquerading as ascariasis. J Clin Neurosci. 2005. 12: 966-7
51. Guthe SP, Pravin S, Darade P, Velho V. Silent migration of ventriculoperitoneal shunt per anus in a child: Management and review of literature. Asian J Neurosurg. 2018. 13: 446-8
52. Handa R, Kale R, Harjai MM. Unusual complication of ventriculoperitoneal shunt: Anal extrusion. Med J Armed Forces India. 2007. 63: 82-4
53. Hasan A, Sharma S, Chopra S, Purohit DK. Anal extrusion of ventriculoperitoneal shunt: A report of two cases and review of literature. J Pediatr Neurosci. 2018. 13: 8-12
54. Hidayat I, Syahputra DA, Isa MM. Unusual migration of distal ventriculoperitoneal shunt to Vagina via fallopian tube: A case report. Ann Med Surg (Lond). 2021. 63: 102158
55. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011. 343: d5928
56. Ibebuike KE. Extrusion of ventriculoperitoneal shunt cathether through a herniotomy wound in an infant: Case report and review of literature. Niger J Clin Pract. 2018. 21: 1542-7
57. Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009. 106: 20388-93
58. Joubert MJ, Stephanov S. Extrusion of peritoneal catheter through the mid-lumbar region. An unusual complication of ventriculo-peritoneal shunt. Surg Neurol. 1983. 19: 120-1
59. Kahle KT, Kulkarni AV, Limbrick DD, Warf BC. Hydrocephalus in children. Lancet. 2016. 387: 788-99
60. Kano T, Kawauchi H. Fibrous encapsulation of the peritoneal catheter in peritoneal shunt: Case report. Surg Neurol Int. 2017. 8: 132
61. Kanojia R, Sinha SK, Rawat J, Wakhlu A, Kureel S, Tandon R. Unusual ventriculoperitoneal shunt extrusion: Experience with 5 cases and review of the literature. Pediatr Neurosurg. 2008. 44: 49-51
62. Kataria R, Sinha VD, Chopra S, Gupta A, Vyas N. Urinary bladder perforation, intra-corporeal knotting, and per-urethral extrusion of ventriculoperitoneal shunt in a single patient: Case report and review of literature. Childs Nerv Syst. 2013. 29: 693-7
63. Kella N, Rathi PK, Qureshi MA. Umbilical perforation: A rare complication of entriculoperitoneal shunt. J Coll Physicians Surg Pak. 2008. 18: 644-5
64. Kelly PD, Yengo-Kahn AM, Naftel RP. The survival of reimplanted shunts following externalization: a single-institution cohort study. J Neurosurg Pediatr. 2021. 27: 382-90
65. Korulmaz A, Alakaya M, Kaya S, Hamzaoglu V, Tezol Ö Arslanköylü AE. A rare cause of vaginal foreign body: Ventriculoperitoneal shunt migration. J Pediatr Neurosci. 2019. 14: 109
66. Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: A prospective study of risk factors. J Neurosurg. 2001. 94: 195-201
67. Kumar B, Sharma SB, Singh DK. Extrusion of ventriculoperitoneal shunt catheter. Indian J Pediatr. 2010. 77: 336
68. Lee CH, Tseng SH, Chen Y. Ileal perforation and transanal protrusion of the peritoneal tube in a boy with a ventriculoperitoneal shunt and literature review. Formosan J Surg. 2015. 48: 209-13
69. Lee SH, Kong DS, Seol HJ, Shin HJ. Endoscopic third ventriculostomy in patients with shunt malfunction. J Korean Neurosurg Soc. 2011. 49: 217-21
70. Lotfinia I, Tubbs S, Mahdkhah A. Vaginal extrusion of a ventriculoperitoneal shunt: A case report and review of literature. J Pediatr Adolesc Gynecol. 2017. 30: e23-5
71. Low SW, Sein L, Yeo TT, Chou N. Migration of the abdominal catheter of a ventriculoperitoneal shunt into the mouth: A rare presentation. Malays J Med Sci. 2010. 17: 64-7
72. Mandhan P, Wong M, Samarakkody U. Laparoendoscopic removal of peroral extrusion of a ventriculoperitoneal shunt. Asian J Endosc Surg. 2015. 8: 95-7
73. Marino M, Phillips C. Methicillin-resistant Staphylococcus aureus meningitis from transanal migration of a ventriculoperitoneal Shunt. J Emerg Med. 2019. 57: e81-4
74. Moghul D, Rahim MT. Protrusion of VP shunt through anus a rare complication after shunt insertion at french medical institute for children (FMIC), Kabul, Afghanistan (case report). Int J Gastroenterol Hepatol Transplant Nutr. 2016. 1: 70
75. Mutlu M, Kader Ş, Aslan Y, Yazar U, İmamoğlu M. An acute complication of ventriculoperitoneal shunt with bladder perforation and extrusion through the Urethra in a Newborn: Case report and review of the literature. Pediatr Neurosurg. 2015. 50: 264-9
76. Nagulic M, Djordjevic M, Samardzic M. Peritoneo-vulvar catheter extrusion after shunt operation. Childs Nerv Syst. 1996. 12: 222-3
77. Odebode TO. Jejunal perforation and peroral extrusion of a peritoneal shunt catheter. Br J Neurosurg. 2007. 21: 235-6
78. Mohta A, Jagdish S. Spontaneous anal extrusion of ventriculoperitoneal shunt. Afr J Paediatr Surg. 2009. 6: 71-2
79. Oktay K, Erkoc YS, Ethemoglu KB, Olguner SK, Sarac ME. Spontaneous extrusion of ventriculoperitoneal shunt catheter through the right lumbar region: A case report and review of the literature. Pediatr Neurosurg. 2015. 50: 336-8
80. Paff M, Alexandru-Abrams D, Muhonen M, Loudon W. Ventriculoperitoneal shunt complications: A review. Interdiscip Neurosurg. 2018. 13: 66-70
81. Pan P. Outcome analysis of ventriculoperitoneal shunt surgery in pediatric hydrocephalus. J Pediatr Neurosci. 2018. 13: 176-81
82. Panigrahi S, Mishra SS, Das S, Tripathy L, Pattajoshi AS. Spontaneous extrusion of peritoneal catheter of ventriculoperitoneal shunt through the intact abdominal wall: Report of two cases. J Pediatr Neurosci. 2012. 7: 228-30
83. Pant N, Singh S, Singh G, Kumar A, Rai RK, Rawat J. The wandering ventriculoperitoneal shunt and the scope of its salvage. Childs Nerv Syst. 2021. 37: 2613-8
84. Park CK, Wang KC, Seo JK, Cho BK. Transoral protrusion of a peritoneal catheter: A case report and literature review. Childs Nerv Syst. 2000. 16: 184-9
85. Patel CD, Matloub H. Vaginal perforation as a complication of ventriculoperitoneal shunt. Case report. J Neurosurg. 1973. 38: 761-2
86. Pohlman GD, Wilcox DT, Hankinson TC. Erosive bladder perforation as a complication of ventriculoperitoneal shunt with extrusion from the urethral meatus: Case report and literature review. Pediatr Neurosurg. 2011. 47: 223-6
87. Prasad VS, Krishna AM, Gupta PK. Extrusion of peritoneal catheter of ventriculoperitoneal shunt through the urethra. Br J Neurosurg. 1995. 9: 209-10
88. Rekate HL. Shunt-related headaches: The slit ventricle syndromes. Childs Nerv Syst. 2008. 24: 423-30
89. Romuald K, Dominique NO, Guy V. Transoral migration of the inferior end of a peritoneal catheter: A rare complication of ventriculoperitoneal shunt. Int J Sci Res. 2016. 7: 1629-32
90. Şahin MH, Temtek U. Enterococcus gallinarum group meningitis after transanal migration of the ventriculoperitoneal shunt: A pediatric case report. Childs Nerv Syst. 2023. 39: 1093-6
91. Sarkari A, Borkar SA, Mahapatra AK. Anal extrusion of migrated ventriculo-peritoneal shunt catheter: An unusual complication and review of literature. Asian J Neurosurg. 2016. 11: 459
92. Shahsavaran S, Kermani HR, Keikhosravi E, Nejat F, El Khashab M. Ventriculoperitoneal shunt migration and coiling: A report of two cases. J Pediatr Neurosci. 2012. 7: 114-6
93. Sharifian A, Abdollahi A, Maddah G, Anaraki F, Alvandipour M, Abbasi Sahebi M. Spontaneous transanal protrusion of ventriculoperitoneal catheter: A case report. Acta Med Iran. 2013. 51: 135-8
94. Silva Neto AR, Bezerra MJ, Farias MC, Câmara RL. Unusual extrusion of ventriculoperitoneal shunt. Acta Neurochir (Wien). 2011. 153: 203-4
95. Simon TD, Schaffzin JK, Stevenson CB, Willebrand K, Parsek M, Hoffman LR. Cerebrospinal fluid shunt infection: Emerging paradigms in pathogenesis that affect prevention and treatment. J Pediatr. 2019. 206: 13-9
96. Sridhar K, Sharma BS, Kak VK. Spontaneous extrusion of peritoneal catheter through intact abdominal wall. Clin Neurol Neurosurg. 1988. 90: 373-5
97. Tamlikha A, Shukri F, Zahari Z. Transoral migration of ventriculoperitoneal shunt: A rare presentation. Gazi Med J. 2020. 32: 131-4
98. Teegala R, Kota LP. Unusual complications of ventriculo peritoneal shunt surgery. J Neurosci Rural Pract. 2012. 3: 361-4
99. Thiong’o GM, Luzzio C, Albright AL. Ventriculoperitoneal shunt perforations of the gastrointestinal tract. J Neurosurg Pediatr. 2015. 16: 36-41
100. Tully HM, Dobyns WB. Infantile hydrocephalus: A review of epidemiology, classification and causes. Eur J Med Genet. 2014. 57: 359-68
101. Turkis OF, Karadag A, Middlebrooks EH, Senoglu M. Anal extrusion of a ventriculoperitoneal shunt. J Coll Physicians Surg Pak. 2019. 29: 478-80
102. Utmanzai S khan, Jamal T, Khalil AA, Ali IM, Ali M. Extrusion of the peritoneal catheter of ventriculoperitoneal shunt through the rectum. Pak J Neurol Surg. 2022. 26: 299-302
103. Vanaclocha V, Sáiz-Sapena N, Leiva J. Shunt malfunction in relation to shunt infection. Acta Neurochir (Wien). 1996. 138: 829-34
104. Vankipuram S, Jaiswal S, Jaiswal M, Bajaj A, Chandra A, Ojha BK. Spontaneous umbilical csf fistula due to migration of the peritoneal end of vp shunt: A case report and review of pathogenesis. J Pediatr Neurosci. 2017. 12: 285-7
105. Voronovich ZA, Albright AL. Enterocutaneous fistula in the setting of ventriculoperitoneal shunt extrusion through the skin and perforation through the small bowel. J Neurosurg Pediatr. 2014. 14: 340-3
106. Vuyyuru S, Ravuri SR, Tandra VR, Panigrahi MK. Anal extrusion of a ventriculo peritoneal shunt tube: Endoscopic removal. J Pediatr Neurosci. 2009. 4: 124-6
107. Xia Y, He F, Ren Z, Wang C. Extrusion of the distal catheter from the umbilicus: A case report of a rare complication after ventriculoperitoneal shunt and its management. Front Pediatr. 2020. 8: 228
108. Zhang J, Qu C, Wang Z, Wang C, Ding X, Pan S. Improved ventriculoatrial shunt for cerebrospinal fluid diversion after multiple ventriculoperitoneal shunt failures. Surg Neurol. 2009. 72: S29-33 discussion S33-4