- Department of Complex Spinal Surgery, Salford Royal NHS Foundation Trust, Stott Lane, Manchester, United Kingdom.
DOI:10.25259/SNI_315_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Riaz Mohammed, Maggie Lee, Shrijit Panikkar, Naveed Yasin, Kamran Hassan, Saeed Mohammad. Vertebral body cemented stents combined with posterior stabilization in the surgical treatment of metastatic spinal cord compression of the thoracolumbar spine. 25-Jul-2020;11:210
How to cite this URL: Riaz Mohammed, Maggie Lee, Shrijit Panikkar, Naveed Yasin, Kamran Hassan, Saeed Mohammad. Vertebral body cemented stents combined with posterior stabilization in the surgical treatment of metastatic spinal cord compression of the thoracolumbar spine. 25-Jul-2020;11:210. Available from: https://surgicalneurologyint.com/surgicalint-articles/10154/
Abstract
Background: Extensile interventions to provide anterior spinal column support in metastatic spinal cord compression (MSCC) surgery incur added morbidity in this surgically frail group of patients. We present our preliminary results of posterior spinal decompression and stabilization coupled with vertebral body cemented stents for anterior column support in MSCC.
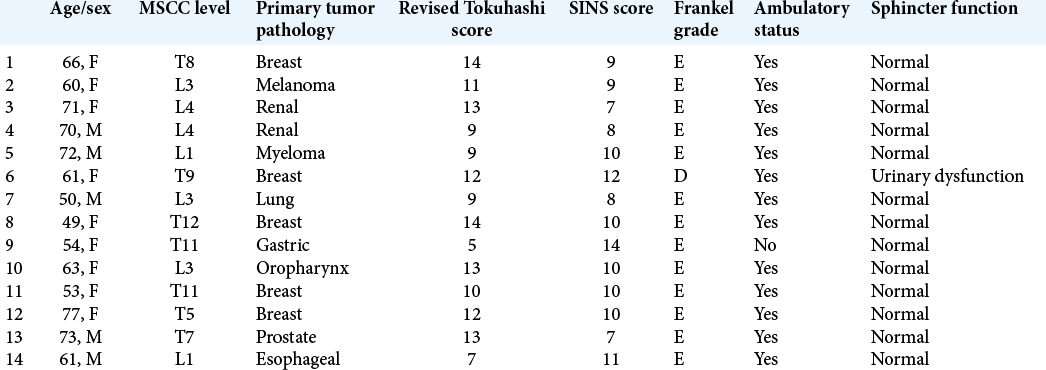
Methods: Fourteen patients underwent posterior spinal decompression and pedicle screw construct along with vertebral body stenting (VBS) technique for reconstruction and augmentation of the vertebral body. The primary in all except one was solid organ malignancy and 10 patients (71%) were treatment naïve. The mean revised Tokuhashi score was 10.7 ± 2.7 and the mean spinal instability neoplastic score was 9.6 ± 1.9. All vertebral body lesions were purely lytic and were associated with a cortical defect in the posterior wall.
Results: A mean 5.3 ± 2.7 ml low-viscosity polymethyl methacrylate bone cement was injected within the stent at each compression level. No cement extrusion posteriorly was noted in any case from intraoperative fluoroscopy or postoperative radiographs. Five patients died at a mean 6.8 months (range 1–15 months), while the remaining patients have a mean survival of 18 months. Neither further revision surgical intervention nor any neurological deterioration was noted in any patient, who all continued to be ambulatory. The mean postoperative Core Outcome Measures Index score for 11 patients was 4.03 (standard deviation 3.11, 95% confidence interval (1.93–6.12).
Conclusion: In lytic vertebral body lesions with posterior wall erosions, cemented VBS technique adds to the surgical armamentarium in MSCC surgery showing promising early results without added complications.
Keywords: Bone cement, Metastatic spinal cord compression, Posterior stabilization, Vertebral augmentation, Vertebral body stent
INTRODUCTION
Standard surgical option in metastatic spinal cord compression (MSCC) involves decompression and spinal stabilization with the aims being to improve pain, restore stability, and address neurological dysfunction.[
There are reports of tumor-induced spinal instability being stabilized by percutaneous pedicle screw-assisted cement augmentation of vertebral column.[
MATERIALS AND METHODS
A pathway and team exist where all patients with MSCC will be discussed with a surgical team which is coordinated through the regional oncology hospital as per the established guidelines.[
Surgical technique
All patients have the procedure under total intravenous anesthesia with antibiotic prophylaxis and intraoperative cell salvage. Patients are placed prone and all pressure areas are well padded, with measures taken to reduce intrathoracic and abdominal pressures.
Midline posterior subperiosteal release approach is performed and pedicle screws inserted at appropriate landmarks under fluoroscopy guidance as needed in the adjacent vertebral segments. Depending on the bone quality and preoperative imaging, we prefer to use fenestrated, cannulated pedicle screws to insert cement to aid the fixation. Posterior decompression is performed with laminectomy, flavectomy, and pedicle excision at the level of cord compression. A connecting rod is applied unilaterally to guard against causing thecal injury through movement before decompression.
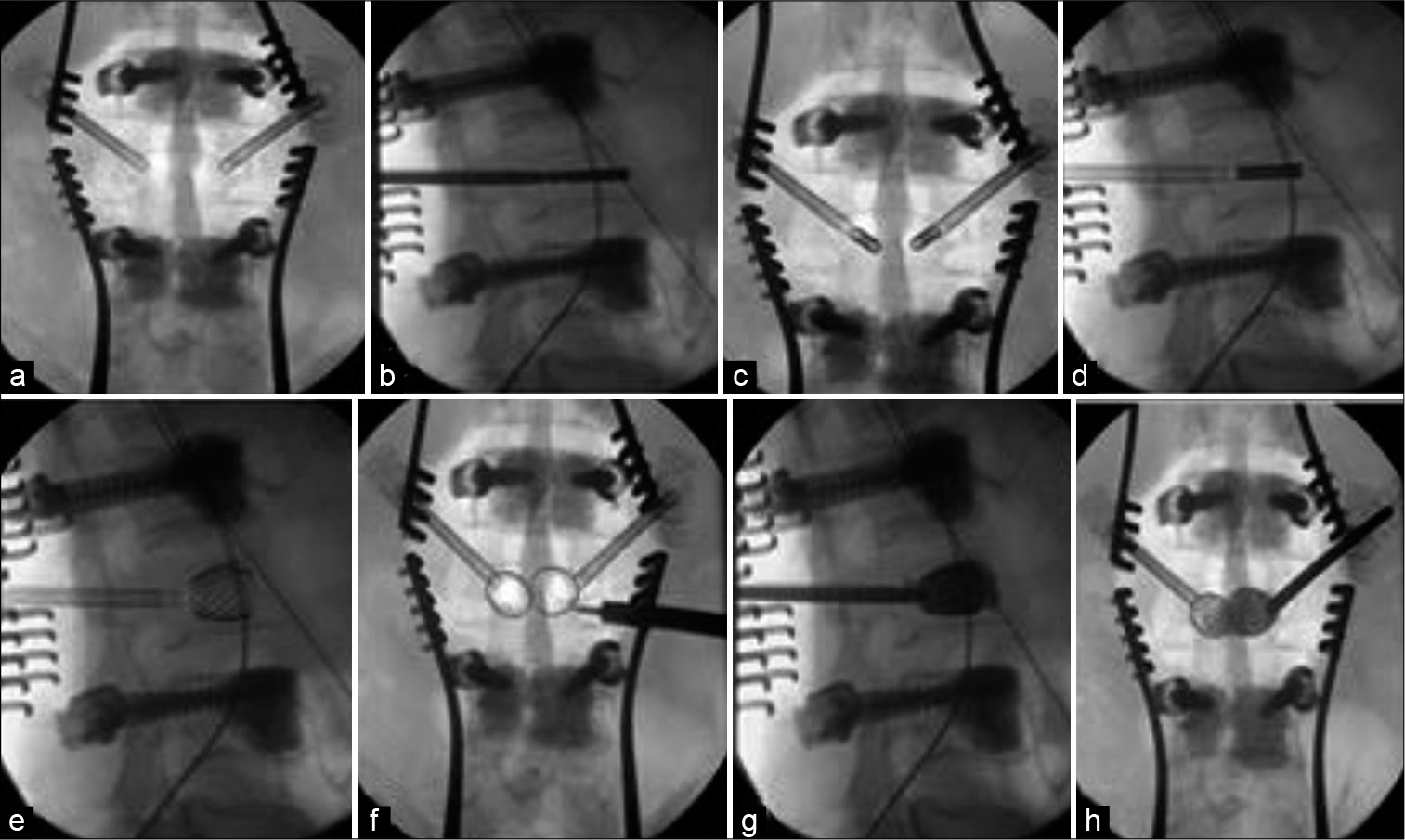
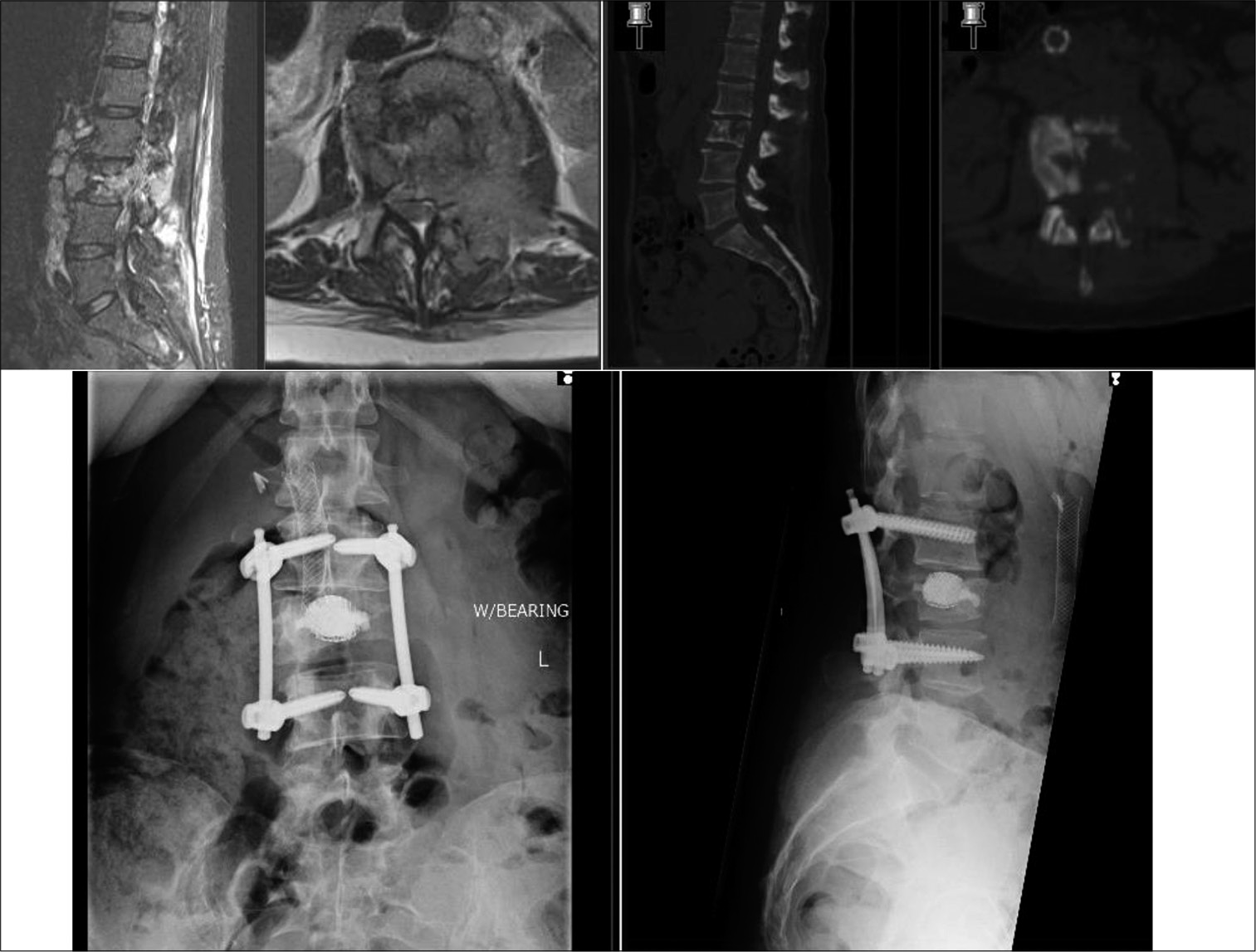
At this point, the decision is made to proceed with vertebral body stent (VBS, DepuySynthes, Switzerland). A cannulated 4.7 mm access kit trocar is inserted through transpedicular route under fluoroscopy and seated approximately 3 mm into the vertebral body [
The balloon catheter with the stent attachment is inserted under lateral fluoroscopy and should ideally be within 5 mm and parallel to the superior end plate of the vertebra [
Cement leakage is a significant complication that can result in paralysis or even death. If cement leakage is observed, it is essential to stop injection and attempt to reposition the needle direction; wait for cement to harden before injecting further; or to completely abandon further injection. We leave the injection syringes attached to the system to prevent any backfilling of the working sleeve. The working sleeve and the injection needle are then removed, preceded by an 180° turn to detach itself from any adherent cement, within the working time of the curing process of bone cement.
The posterior stabilization construct is then finally secured and the wound closed in layers. Patients are managed with early mobilization and discharged after satisfactory recovery for further treatment appropriate for their primary tumor.
RESULTS
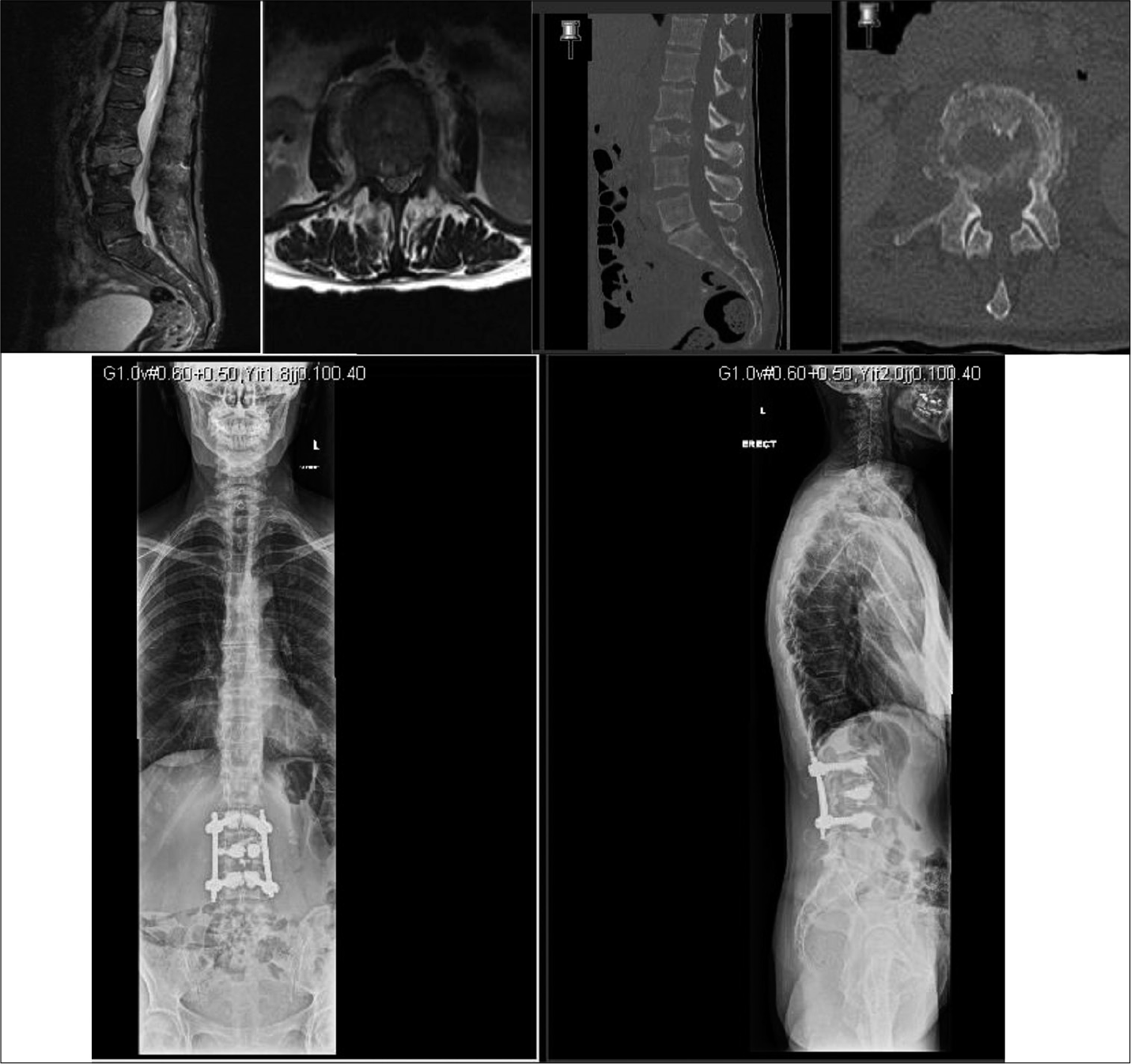
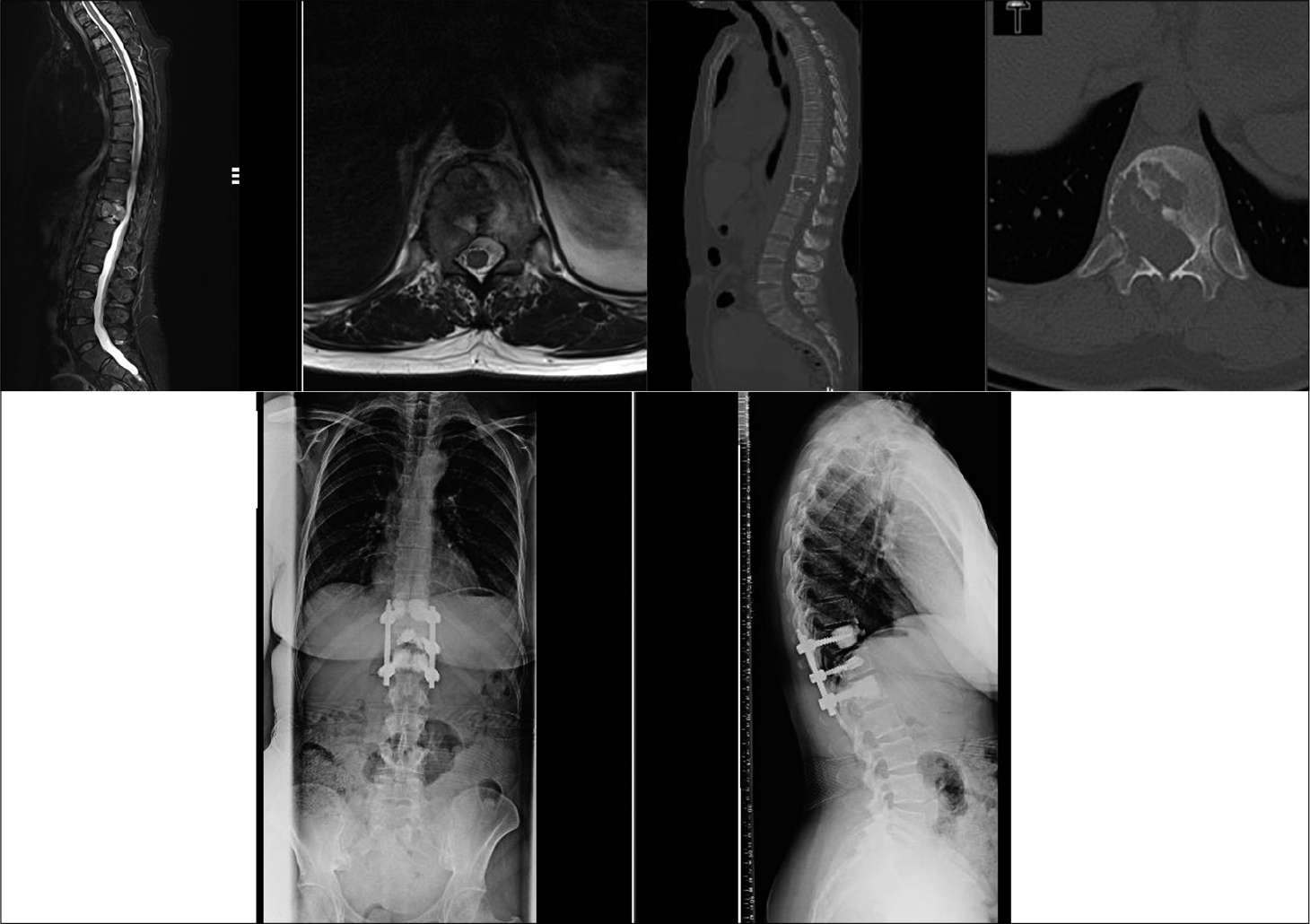
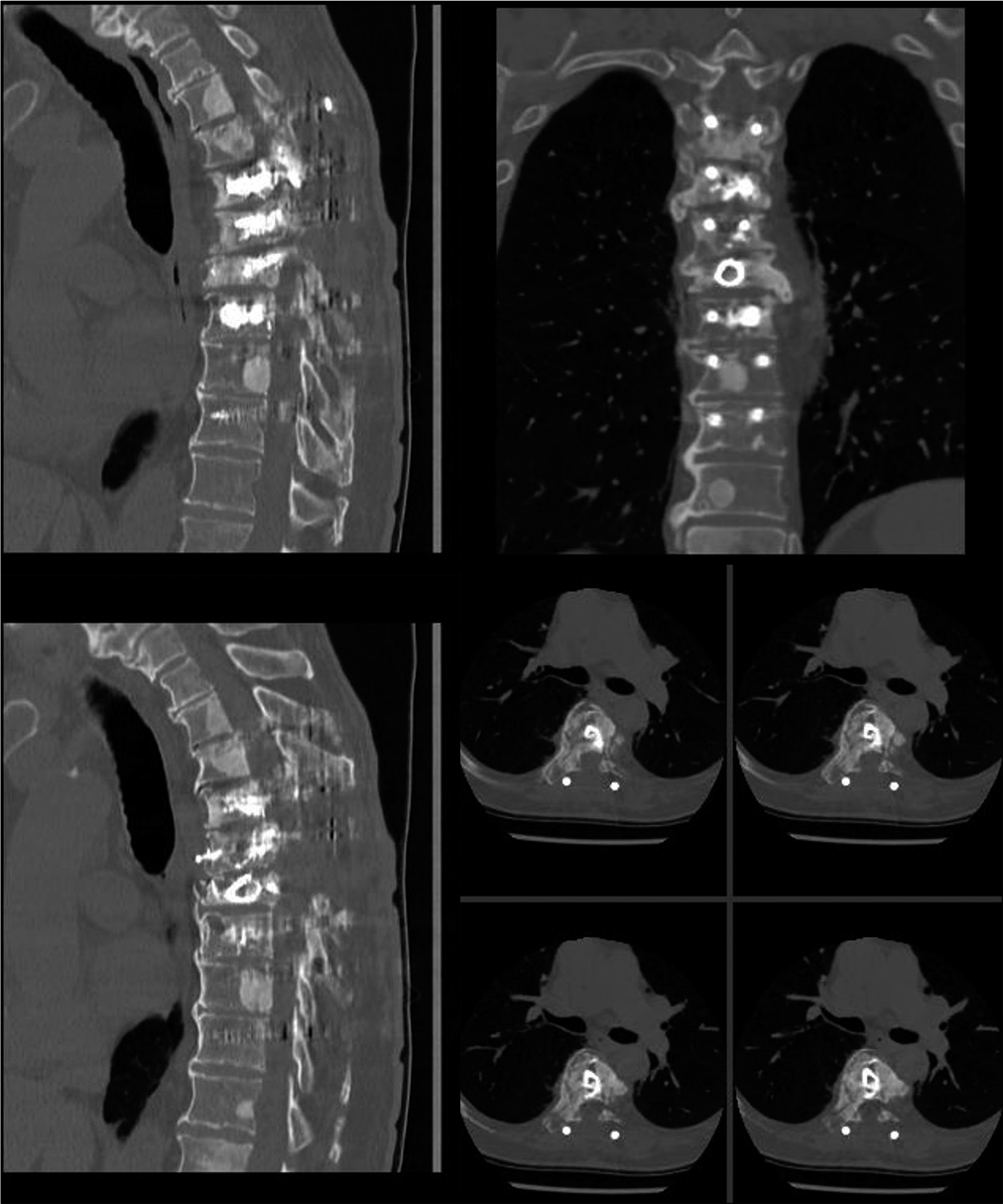
We analyzed 14 patients who underwent posterior spinal decompression and pedicle screw construct along with VBS technique for reconstruction and augmentation of the vertebral body [
A mean 5.3 ±2.7 ml low-viscosity PMMA bone cement was injected within the stent at each compression level. No cement extrusion posteriorly was noted in any case from intraoperative fluoroscopy or postoperative radiographs. There were no procedural stent-related issues except in three cases were the stent failed to fully inflate. Eight patients also had cement insertion into adjacent level vertebral bodies through fenestrated pedicle screws for added construct stability. The fixation construct was kept within one segmental level on either side of the diseased vertebra in six patients, while the mean fixation level for the whole cohort was 3.3 segmental levels (range 2–6).
Five patients died at a mean 6.8 months (range 1–15 months), while the remaining patients have a mean survival of 18 months at the time of the study. Neither further revision surgical intervention nor any neurological deterioration was noted in any patient. All patients remained ambulatory after surgery and none had any neurological deterioration pertaining to the MSCC level at subsequent follow-up reviews [
Core Outcome Measures Index (COMI) scores evaluate the patient’s pain, functionality, generic health status or well- being, disability, and satisfaction.[
DISCUSSION
There are reports in the literature of pedicle screw fixation combined with cement augmentation for spinal metastasis causing instability[
There is paucity in the literature regarding cement augmentation techniques in spinal metastasis, as majority of evidence is in osteoporotic compression fractures. VP though has similar pain relief in spinal tumors as kyphoplasty,[
Despite the use in the past decade for osteoporotic spinal fractures, VBS has not been indicated for use in spinal tumors. The potential advantage of a reliable cement filled stent that restores vertebral body height aids in anterior column stability and lesser potential for cement leakages while avoiding the extensile anterior surgery in this frail MSCC, patient group has prompted us in using VBS- assisted posterior spinal decompression and stabilization. Our indications are lytic or mixed pattern of spinal metastasis and careful surgical planning is crucial for good surgical outcomes. Posterior cortical breach is not a contraindication and by extension neither is involvement of the vertebral end plates. To prevent stent from protruding back, peroperative imaging is used to position and size the stent as per the space available. As the stent is filled up with cement in a controlled manner, the cement infiltrates the surrounding cancellous bone, forming a micro-interlock at the bone-cement interface. The resultant fixation aids to anchor the stent in its inserted position. We have not come across stent migration in postoperative radiographs in our study [
The procedure is not without pitfalls, primarily associated with cement leakages, number of stents used, and incomplete stent deployment. The cement augmentation of the stent is performed with utmost care under fluoroscopy guidance, so as to avoid cement leakages. Cement can still leak if one is not careful in timing it. It is essential to meticulously use the stent to form a shell with the cement as far as possible. Compared to VP group (42.1%), the frequency of cement leakage after VBS was reported to be 25.5% in a series of patients with vertebral osteoporotic fractures without neurological deficit.[
Although bilateral stents may support the anterior column symmetrically, in tumor surgery, this is neither always needed nor feasible. Unilateral stents are advisable in vertebral lytic lesions that involve only one half of the vertebral body, in small-sized vertebral bodies or operative technicalities preclude bilateral stent insertion. The stent is directed more across the midline in these cases [
Recent literature has shown promising results of VBS in spinal metastasis. Cianfoni et al. have shown VBS use as anterior augment in neoplastic osteolysis[
Our preliminary report on VBS for anterior column support in spinal metastasis with posterior cortical breach along posterior decompression and stabilization is the largest report of this application. We rely on careful surgical technique for stent and cement insertion in vertebral body lesions destroyed by tumor that otherwise would need a longer construct or a massive surgical trauma. Extensile surgical interventions are fraught with significant complications that can impair the remaining life of the patient. The aim is to keep the fixation short, as a means to decompress and stabilize the spine to improve the patient’s quality of life.
CONCLUSION
The addition of VBS during posterior decompression and stabilization surgery for MSCC achieved a stable fixation construct without the need for extensive anterior surgery to support the anterior spinal column. Stent expansion was seen fairly consistently allowing cement insertion and no cement leakages were noted with careful technique. We believe this preliminary report of VBS in MSCC surgery adds to the surgical armamentarium with promising early results and without major complications.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Astur N, Avanzi O. Balloon kyphoplasty in the treatment of neoplastic spine lesions: A systematic review. Global Spine J. 2019. 9: 348-56
2. Barzilai O, McLaughlin L, Lis E, Reiner AS, Bilsky MH, Laufer I. Utility of cement augmentation via percutaneous fenestrated pedicle screws for stabilization of cancer-related spinal instability. Oper Neurosurg (Hagerstown). 2019. 16: 593-9
3. Boszczyk B. Volume matters: A review of procedural details of two randomised controlled vertebroplasty trials of 2009. Eur Spine J. 2010. 19: 1837-40
4. Bouza C, Lo´pez-Cuadrado T, Cediel P, Saz-Parkinson Z, Amate JM. Balloon kyphoplasty in malignant spinal fractures: A systematic review and meta-analysis. BMC Palliat Care. 2009. 8: 12-
5. Cianfoni A, Distefano D, Isalberti M, Reinert M, Scarone P, Kuhlen D. Stent-screw-assisted internal fixation: The SAIF technique to augment severe osteoporotic and neoplastic vertebral body fractures. J Neurointerv Surg. 2019. 11: 603-9
6. Cianfoni A, Distefano D, Pravatà E, Espeli V, Pesce G, Mordasini P. Vertebral body stent augmentation to reconstruct the anterior column in neoplastic extreme osteolysis. J Neurointerv Surg. 2019. 11: 313-8
7. Cianfoni A, Distefano D, Scarone P, Pesce GA, Espeli V, La Barbera L. Stent screw-assisted internal fixation (SAIF): Clinical report of a novel approach to stabilizing and internally fixating vertebrae destroyed by malignancy. J Neurosurg Spine. 2019. 32: 507-18
8. Deyo R, Battie M, Beurskens A, Bombardier C, Croft P, Koes B. Outcome measures for low back pain research. A proposal for standardized use. Spine (Phila Pa 1976). 1998. 23: 2003-13
9. Fisher CG, DiPaola CP, Ryken TC, Bilsky MH, Shaffrey CI, Berven SH. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine oncology study group. Spine (Phila Pa 1976). 2010. 35: E1221-9
10. Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrar K. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003. 98: 21-30
11. Gerszten PC, Monaco EA. Complete percutaneous treatment of vertebral body tumors causing spinal canal compromise using a transpedicular cavitation, cement augmentation, and radiosurgical technique. Neurosurg Focus. 2009. 27: E9-
12. Halpin RJ, Bendok BR, Liu JC. Minimally invasive treatments for spinal metastases: Vertebroplasty, kyphoplasty, and radiofrequency ablation. J Support Oncol. 2004. 2: 339-51
13. Metastatic Spinal Cord Compression Overview. NICE Guidance. Available from: https://www.pathways.nice.org.uk/pathways/metastatic-spinal-cord-compression [Last accessed on 2020 May 20].
14. Network Flowchart/Pathway. Available from: https://www.christie.nhs.uk/patients-and-visitors/services/metastatic-spinal-cord-compression-mscc/information-for-professionals/network-flowchartpathway[Last accessed on 2020 May 20].
15. Mannion A, Elfering A, Staerkle R, Junge A, Grob D, Semmer N. Outcome assessment in low back pain: How low can you go?. Eur Spine J. 2005. 14: 1014-26
16. Martín-López JE, Pavón-Gómez MJ, Romero-Tabares A, Molina-López T. Stentoplasty effectiveness and safety for the treatment of osteoporotic vertebral fractures: A systematic review. Orthop Traumatol Surg Res. 2015. 101: 627-32
17. Mendel E, Bourekas E, Gerszten P, Golan JD. Percutaneous techniques in the treatment of spine tumors: What are the diagnostic and therapeutic indications and outcomes?. Spine (Phila Pa 1976). 2009. 34: S93-100
18. Moussazadeh N, Rubin DG, McLaughlin L, Lis E, Bilsky MH, Laufer I. Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J. 2015. 15: 1609-17
19. Quraishi NA, Gokaslan ZL, Boriani S. The surgical management of metastatic epidural compression of the spinal cord. J Bone Joint Surg Br. 2010. 92: 1054-60
20. Rose PS, Buchowski JM. Metastatic disease in the thoracic and lumbar spine: Evaluation and management. J Am Acad Orthop Surg. 2011. 19: 37-48
21. Rotter R, Martin H, Fuerderer S, Gabl M, Roeder C, Heini P. Vertebral body stenting: New method for vertebral augmentation versus kyphoplasty. Eur Spine J. 2010. 19: 916-23
22. Schroeder JE, Ecker E, Skelly AC, Kaplan L. Cement augmentation in spinal tumors: A systematic review comparing vertebroplasty and kyphoplasty. Evid Based Spine Care J. 2011. 2: 35-43
23. Thaler M, Lechner R, Nogler M, Gstöttner M, Bach C. Surgical procedure and initial radiographic results of a new augmentation technique for vertebral compression fractures. Eur Spine J. 2013. 22: 1608-16
24. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005. 30: 2186-91
25. Weitao Y, Qiqing C, Songtao G, Jiaqiang W. Open vertebroplasty in the treatment of spinal metastatic disease. Clin Neurol Neurosurg. 2012. 114: 307-12
26. Zhou Z, Wang Y, Sun Z, Qian Z. Safety of cement distribution patterns in metastatic vertebral tumors: A retrospective study. Med Sci Monit. 2019. 25: 7228-34