- Spinal Surgery Unit - Trauma and Orthopaedics, The Walton Centre NHS foundation Trust, Lower Lane, Liverpool, United Kingdom.
Correspondence Address:
Sai Gautham Balasubramanian, Spinal Surgery Unit - Trauma and Orthopaedics, The Walton Centre NHS foundation Trust, Lower Lane, Liverpool, United Kingdom. ;
DOI:10.25259/SNI_692_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aditya Anand Dahapute, Sai Gautham Balasubramanian, Prokopis Annis. White cord syndrome following posterior decompression and fusion for severe OPLL and an acute traumatic cervical injury – A case report and review of literature. 28-Oct-2022;13:501
How to cite this URL: Aditya Anand Dahapute, Sai Gautham Balasubramanian, Prokopis Annis. White cord syndrome following posterior decompression and fusion for severe OPLL and an acute traumatic cervical injury – A case report and review of literature. 28-Oct-2022;13:501. Available from: https://surgicalneurologyint.com/surgicalint-articles/11953/
Abstract
Background: White cord syndrome (WCS) refers to the observation of intramedullary hyperintensity due to edema/ischemia and swelling on postoperative T2-weighted MRI sequences in the setting of unexplained neurological deficits after cervical spinal cord decompression. Pathophysiologically, WCS/reperfusion injury (RPI) occurs due to oxygen derived free radicals as a result of acute reperfusion or direct trauma from blood flow itself. Intraoperative neurophysiologic monitoring (IONM) can give early warning and detect neurologic deficits. Here, we are presenting a case of a patient who had a chronic severe ossification of posterior longitudinal ligament (OPLL) of cervical cord, underwent decompressive surgery, and developed quadriplegia postoperatively without any perceptible iatrogenic cord trauma, documented by IONM and postoperative MRI with classical signs of WCS.
Case Description: A 63-year-old male presented with low velocity fall at home followed by quadriparesis. X-ray images on presentation showed C6 fracture and local kyphosis. MRI images showed that there is marked spinal canal stenosis from C2 down to C4 due to OPLL with intrinsic signal changes in the cord. On decompression, motor-evoked potential signals were not present below C4. Immediate postoperative MRI was done to rule out any compressive pathology. MRI showed T2 hyperintensity of the cord at C3 level with cord edema. No evidence of epidural hematoma or other compressive lesion was found and the diagnosis of WCS/RPI was established.
Conclusion: WCS is essentially a diagnosis of exclusion. Very rarely, patients sustain severe/new neurological deficits postoperatively attributed to WCS. Unless, this is confirmed postoperatively with classical MRI signs of intramedullary hyperintensity, the diagnosis should not be invoked.
Keywords: Cervical spine, Diagnosis of exclusion, Ossification of posterior longitudinal ligament, Reperfusion injury of cord, White cord syndrome
INTRODUCTION
Posterior cervical decompression and fusion (PCDF) is a frequently performed technique for cervical spondylotic myelopathy (CSM). New neurologic deficits are rare complications following spine surgery, occurring at a rate <1%.[
White cord syndrome (WCS) has been described as neurodeficit that cannot be explained by these things, which can be caused by an acute reperfusion injury (RPI) of the spinal cord. The so called “WCS” is usually a diagnosis of exclusion as it can be diagnosed only if all the other etiologies have been ruled out. Iatrogenic and postoperative compressive pathologies are still the leading causes of neurological deficits postoperatively. Spinal cord RPI is a rare complication and only few cases are reported in the literature. First described by Chin et al.,[
Ischemia-RPI typically occurs after a cervical decompression surgery, where a chronically compressed spinal cord is injured by reperfusion following decompression. Such an injury occurs due to acute restoration of blood flow to the previously ischemic spinal cord due to long standing compression like ossification of posterior longitudinal ligament (OPLL) in cervical spine. This can occur in various cervical spine surgeries, both anterior and posterior approaches such as anterior cervical decompression and fusion (ACDF), multiple laminectomies, or corpectomy to relieve chronically compressed spinal cord. Pathophysiologically, WCS/RPI occurs due to oxygen-derived free radicals as a result of acute reperfusion or direct trauma from blood flow itself. This, in turn, gives rise to a cascade of reactions such as lipid peroxidation of neuronal membrane and change of architecture of spinal cord resulting in secondary injury.[
Here, we are presenting a case of a patient who had a chronic severe OPLL of cervical cord who underwent decompressive surgery and developed quadriplegia postoperatively without any perceptible iatrogenic technical cord trauma, documented by IONM and postoperative MRI with classical signs of WCS.
CASE PRESENTATION
A 63-year-old male presented to us with low velocity fall at home followed by quadriparesis. On examination, he had upper limb weakness more than lower limb (Right upper limb power 1/5, the left upper limb power 2/5, and power in both lower limbs 3/5). Sensation to catheter tug presents with intact perianal sensation. The hand dexterity was poor in both upper limbs with the upper motor neuron signs in bilateral upper and lower limbs. ATLS protocol was followed when the patient arrived to the emergency department. Cervical spine was immobilized and patient was sent for further investigations like CT scan of whole spine and brain with MRI scan of cervical spine.
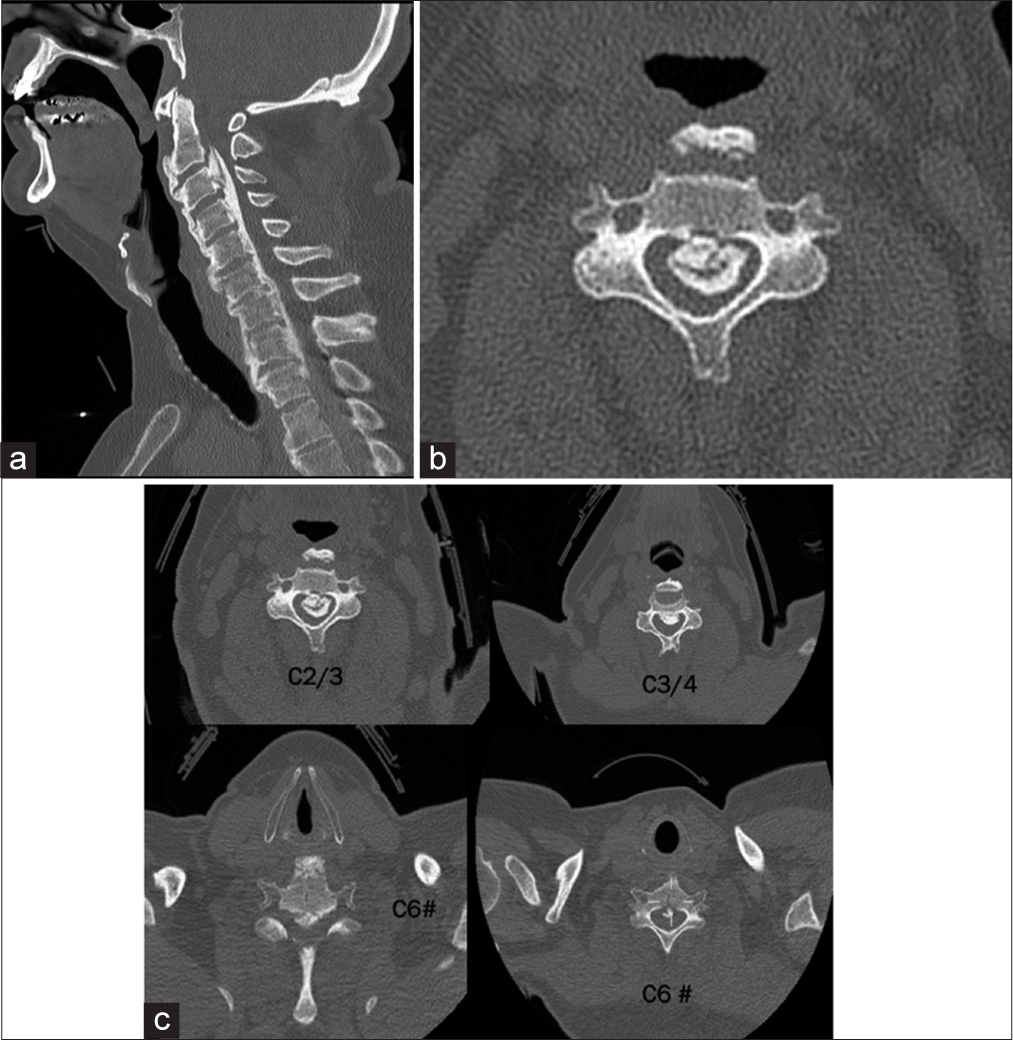
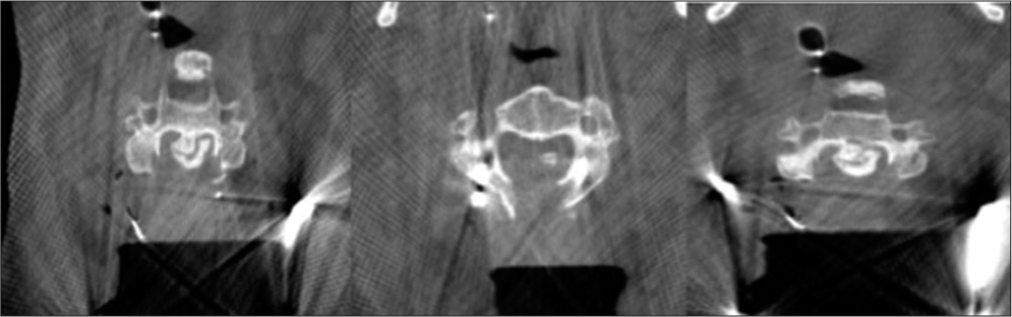
The CT scan demonstrated C6 fracture with ossification of the posterior longitudinal ligament in addition to diffuse idiopathic skeletal hyperostosis with anterior osteophytosis. CT picture showed florid OPLL from C2 to C4 with horizontal fracture through the entire width of the C6 vertebral body which extended posteriorly to bilateral facet joints without any dislocation/displacement. There is also calcification of the longitudinal ligament all the way down to T3. However, it was particularly severe from C2 to C4. There was also suggestion of fracture of the ossified PLL at C3 level [
Figure 1:
CT images demonstrating C6 fracture with ossification of the posterior longitudinal ligament in addition to diffuse idiopathic skeletal hyperostosis with anterior osteophytosis. There is calcification of the longitudinal ligament from C2/3 down to T3. (a) Sagittal, (b) axial at C3 with island of ossification of posterior longitudinal ligament, and (c) CT scan axial at various levels from C2/3 till C6 #: CT scan axial at various levels from C2/3 till C6.
Figure 2:
MRI scan images showing that there is marked spinal canal stenosis from C2 down to C4 due to ossified posterior longitudinal ligament with intrinsic signal changes in the cord. (a) Sagittal scan of cervicothoracic spine, (b) images showing severe cord compression at C3/4 levels, and (c) at C2/3 level. The blue bar in figures 2b and 2c indicates the axial level. 2b axial at C3/4 disc level and 2c axial at C3 vertebral body level. This also corresponds to the area of maximal stenosis and cord compression.
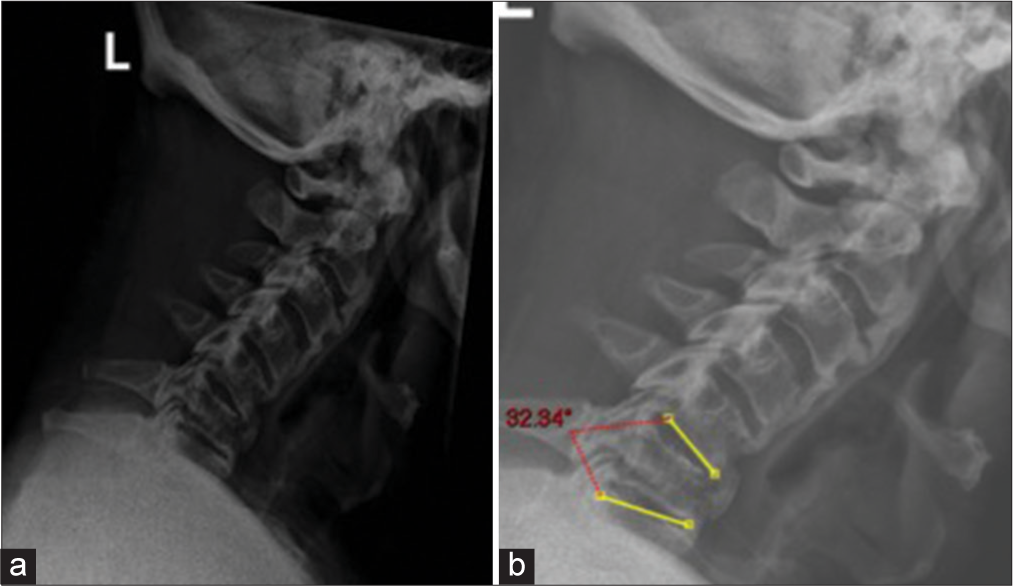
The patient was initially placed in a cervicothoracic brace for temporary stabilization. The patient’s neurology improved after the initial injury, and hence, the decision was made to continue the brace to allow his neurology to improve further and settle at a baseline after multidisciplinary team (MDT) discussion. The plan was to operate him on an elective basis after 7–10 days to allow for complete assessment. However, subsequent standing AP and lateral X-rays showed 32° of local kyphosis without brace with reduction in the width of C6 body through the fracture line [
The plan was to proceed with a posterior cervical decompression from C2 to C7 and instrumented fusion from C2 to T2 under navigation and neuromonitoring. The case was discussed in the local MDT and there was overall agreement about the decision to proceed with surgery and the surgical plan. Despite the fact in the UK use of spinal cord monitoring in cervical myelopathy is not commonly used, we had recognized that the risks were significantly higher in the presence of severe pre-existing OPLL and an unstable fracture in this particular case.
Intraoperative monitoring (IONM) was undertaken using a 32 channels and multimodal XLTEK Protektor intraoperative monitoring recording system. Intraoperative recording modalities consisted of transcranial motor-evoked potentials (MEPs), free running electromyography, and upper limb and lower limb somatosensory-evoked potentials (SSEPs). SSEPs were recorded for all four limbs using stainless steel corkscrew electrodes supine MEP baselines that were recorded before the patient was transferred to the surgical table. The patient was transferred to a prone position and MEP responses repeated and set as baseline.
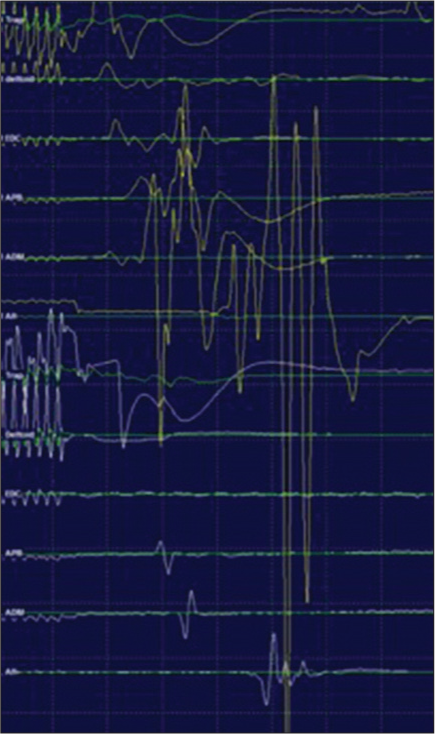
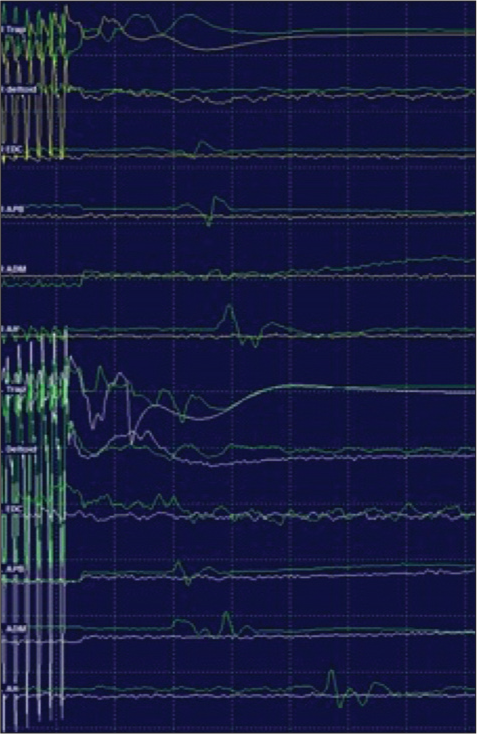
Transcranial MEP amplitudes remained stable during placement of screws from C2 to T2 under navigation. Posterior cervical decompression was carried out with bone cuts using bone scalpel for laminectomy. During decompression, after the laminectomy at C3/4 was completed, transient amplitude changes were noted for the right extensor digitorum communis (EDC), right abductor pollicis brevis (APB), and right abductor digiti minimi (ADM). When the lamina was removed, MEP responses initially augmented for the right trapezius, right deltoid, right EDC, right APB, right ADM, and right abductor hallucis [
Figure 5:
Motor-evoked potential (MEP) abolished below C4, MEPs remained present bilaterally from trapezius, right (yellow), left (white) and abolished below from deltoid, extensor digitorumcommunis, abductor pollicis brevis, adductor digiti minimi, and abductor hallucis. Prone baseline MEPs are displayed in green.
The spinal cord at risk protocol was initiated. A bolus of the decompression was extended higher and lowers including the whole of posterior elements of C2–C7. The mean arterial pressure (MAP) was kept above 85 mmHg for the whole procedure. To assess for any ongoing compression and hardware malposition, we performed an intraoperative CT which showed that the decompression was satisfactory and no evidence of spinal subluxation or screw misplacement [
Five minutes from losing MEPs, the right upper limb SSEPs showed a 50% decrease in amplitude from baseline values with an increase in the sensory threshold (50 mA, 1.0 ms). The left upper limb SSEPs remained with intraoperative baseline values. Bilateral lower limb SSEPs remained stable and within intraoperative baseline values. MEPs remained absent below trapezius during rod placement.
Following the placement of rods and completion of the surgical procedure, MEPs remained absent below trapezius. The right upper limb SSEP showed minimal N20 amplitude reduction (<50%) from intraoperative baseline values, the left upper limb SSEPs were within baseline values, the right lower limb SSEPs remained within intraoperative baseline values, and the left lower limb SSEPs showed a reduction in P37 amplitude of 50% from intraoperative baseline values.
The patient was transferred to ITU intubated with ventilator support, MAP >85, and to continue dexamethasone 8MG twice daily. Postoperatively, he had quadriplegia with C5 sensory level. Immediate postoperative MRI was done to rule out any compressive pathology.
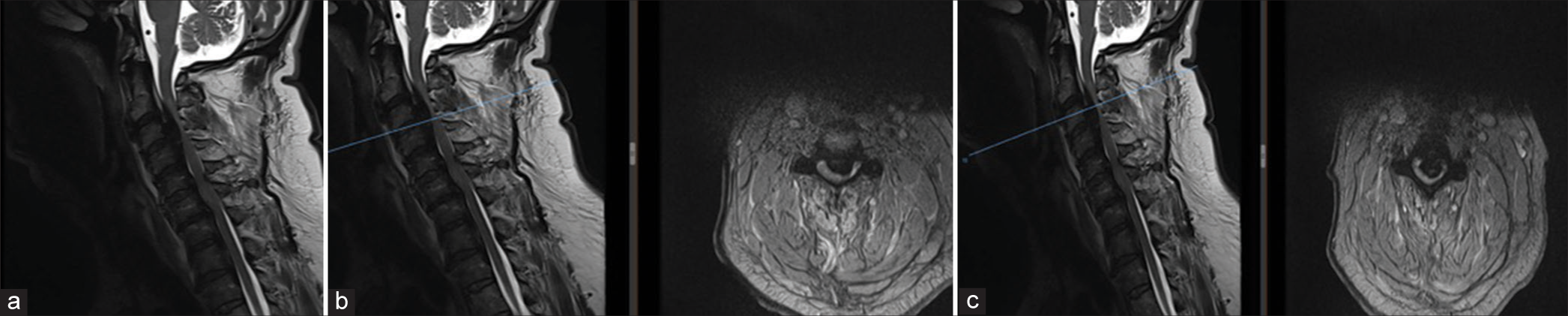
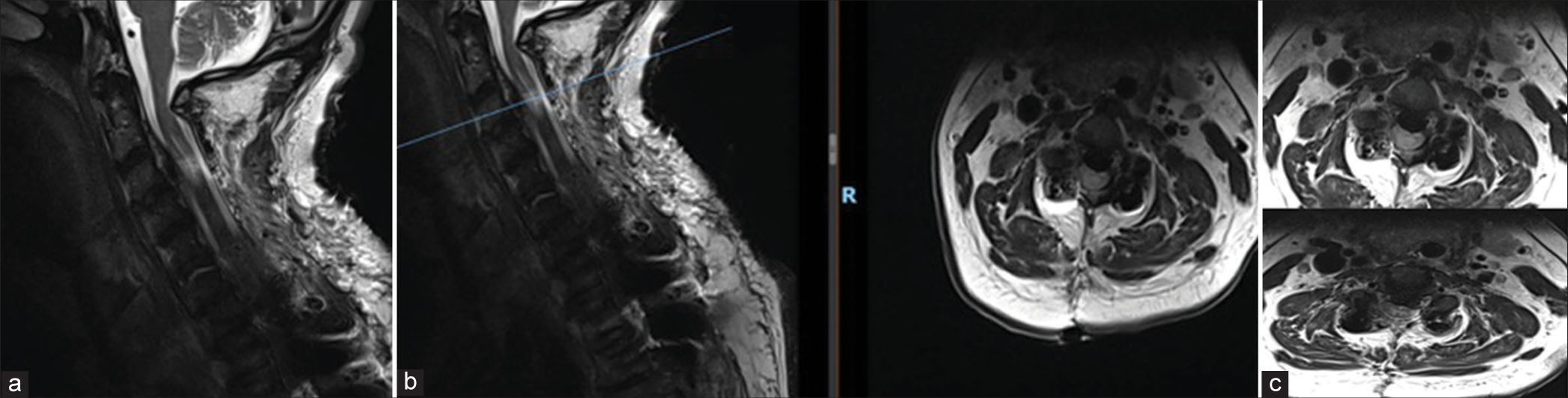
MRI showed T2 intramedullary hyperintensity of the cord at C3 level which appeared expanded with a central syrinx. Cord edema was noted at the C3 level. No evidence of epidural hematoma or other compressive lesion was noticeable at the operative site [
Figure 7:
Immediate postoperative MRI was done to rule out any compressive pathology. (a) MRI showed T2 intramedullary hyperintensity of the cord at C3 level which appeared expanded with a central syrinx. (b) Cord edema was noted at the C3 level. No evidence of epidural hematoma or other compressive lesion at the operative site indicating reperfusion injury. The blue bar in the figure indicates the axial images with maximal intramedullary hyperintensity of the cord and the reperfusion injury which corresponds to C3 level. (c) Axial at C3 and C3/4 Levels showing whitening of the cord (White Cord Syndrome).
Steroids were tapered over a period of 1 week. Tracheostomy was performed and gradual weaning from ventilator support was started. The patient was transferred to inpatient rehabilitation center 6 weeks after surgery with still no improvement in motor strength.
DISCUSSION AND REVIEW OF LITERATURE
WCS is a rare complication of surgery of the spinal cord. This ischemic edematous lesion of the cord is attributed to injury due to immediate reperfusion of the newly-decompressed cord intra- or postoperatively. In the literature, we could find very few cases of WCS/RPI following anterior/ posterior cervical decompressive surgeries. The immediate postoperative MRI findings in all such cases were consistent with the RPI. All such case reports/series involved similar postoperative clinical pictures and MRI images as above.
Chin et al.[
Giammalva et al.[
Bayley et al.[
Jun et al.[
Antwi et al.[
Busack and Eagleton[
Vinodh et al.[
Wiginton et al.[
Study reported by Seichi et al.[
Mathkour et al.[
In our case, the patient had a severe cervical cord compression due to OPLL at C2–4 level. There was a documented fall in MEPs and SSEPs few minutes after decompression without any iatrogenic injury to the cord. There was no demonstrable cause which can cause neurodeficit like implant position and adequate decompression was checked with intraoperative O arm. Furthermore, postoperative MRI was done to exclude any residual compression which demonstrated the classical intramedullary hyperintensity of the cord at the site of maximal cord compression.
Various theories have been postulated for the WCS in the absence of mechanical cord injury, which includes ischemiaRPI, microthrombi, and altered perfusion following decompression. Antwi et al. hypothesized that areas of the cord that are chronically compressed can become ischemic from small artery occlusion.[
Another mechanism or the secondary mechanism of injury is due to the lipid peroxidation of the neuronal membrane with mitochondrial mediated apoptosis of the neuronal cells. In rats, an experimental study was made by mechanical compression of the cord for 6 weeks and MRI was performed pre- and post decompression to look for changes in the cord. This study showed that, after decompression of the spinal cord, increased inflammatory markers, tumor necrosis factor α, and interleukin-1 β11 were observed in conjunction with the reperfusion period.[
At present, IONM is not a routine of practice. However, in our case, it did gave us an alert as there was drop in MEP and SSEP to which we acted promptly by doing further decompression until C2, increasing MAP, steroids, and performing intraoperative imaging with O-arm to make sure adequate implant position and decompression. Furthermore, the signal changes happened post decompression which effectively rules out implant/instrument related cord injury which can play a role medicolegally as well. Unfortunately, it did not make a difference in prognosis of the patient, but it did give us a timely alert to act promptly.
CONCLUSION
WCS is essentially a diagnosis of exclusion. Very rarely, patients sustain severe/new neurological deficits postoperatively attributed to WCS. Unless, this is confirmed postoperatively with classical MRI signs, the diagnosis should not be invoked. IONM can help us detect such an event occurring intraoperatively and help us take appropriate measures in a timely manner. IONM should be the standard of care in such cases of chronic compression and also, it provides a source of permanent record of what exactly happened intraoperatively to prevent medicolegal complications. Understanding WCS/RPI is a valid diagnosis in such circumstances that would be helpful in detailed consenting processes and help prevent an additional decompression surgery for the patient which might, further, increase the risks of mortality and morbidity.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Antwi P, Grant R, Kuzmik G, Abbed K. “White cord syndrome” of acute hemiparesis after posterior cervical decompression and fusion for chronic cervical stenosis. World Neurosurg. 2018. 113: 33-6
2. Bayley E, Boszczyk B, Cheong R, Srivastava A. Major neurological deficit following anterior cervical decompression and fusion: What is the next step?. Eur Spine J. 2015. 24: 162-7
3. Busack CD, Eagleton BE. White cord syndrome causing transient tetraplegia after posterior decompression and fusion. Ochsner J. 2020. 20: 334-8
4. Chin KR, Seale J, Cumming V. “White cord syndrome” of acute tetraplegia after anterior cervical decompression and fusion for chronic spinal cord compression: A case report. Case Rep Orthop. 2013. 2013: 697918
5. Epstein NE. Reperfusion injury (RPI)/white cord syndrome (WCS) due to cervical spine surgery: A diagnosis of exclusion. Surg Neurol Int. 2020. 11: 320
6. Giammalva G, Maugeri R, Graziano F, Gul C, Giugno A, Basile L. White cord syndrome after non-contiguous double-level anterior cervical decompression and fusion (ACDF): A “no reflow phenomenon?. ” Interdiscip Neurosurg. 2017. 7: 47-9
7. Jun DS, Baik JM, Lee SK. A case report: White cord syndrome following anterior cervical discectomy and fusion: Importance of prompt diagnosis and treatment. BMC Musculoskelet Dis. 2020. 21: 157
8. Karadimas SK, Fehlings MG, Yu WR, Satkunendrarajah K, Kallitsis JK, Gatzounis G. A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiol Dis. 2013. 54: 43-58
9. Mathkour M, Werner C, Riffle J, Scullen T, Dallapiazza R, Dumont A. Reperfusion “white cord’’ syndrome in cervical spondylotic myelopathy: Does mean arterial pressure goal make a difference? Additional case and literature review. World Neurosurg. 2020. 137: 194-9
10. Seichi A, Takeshita K, Kawaguchi H, Nakajima S, Akune T, Nakamura K. Postoperative expansion of intramedullary high-intensity areas on T2-weighted magnetic resonance imaging after cervical laminoplasty. Spine (Phila Pa 1976). 2004. 29: 1478-82
11. Vinodh VP, Rajapathy SK, Sellamuthu P, Kandasamy R. White cord syndrome: A devastating complication of spinal decompression surgery. Surg Neurol Int. 2018. 9: 136
12. Wang T, Tian XM, Liu SK, Wang H. YZ, Ding WY. Prevalence of complications after surgery in treatment for cervical compressive myelopathy: A meta-analysis for last decade. Medicine (Baltimore). 2017. 96: e6421
13. Wiginton JG, Brazdzionis J, Mohrdar C, Sweiss R, Lawandy S. Spinal cord reperfusion injury: Case report, review of the literature, and future treatment strategies. Cureus. 2019. 11: e5279