- Department of Radiological Sciences, University of Messina, Messina, Italy
- Department of Experimental Biomedicine and Clinical Neurosciences (BIONEC), University of Palermo, Palermo, Italy
- Department of Human Pathology, Medical Oncology Unit, University of Messina, Messina, Italy
- Department of Neurosurgery, University of Messina, Messina, Italy
Correspondence Address:
Concetta Alafaci
Department of Neurosurgery, University of Messina, Messina, Italy
DOI:10.4103/2152-7806.179429
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Granata F, Morabito R, Grasso G, Alafaci E, Salpietro FM, Alafaci C. A rare case of solitary brain Langerhans cell histiocytosis with intratumoral hemorrhage in a patient affected by Turner syndrome. Surg Neurol Int 30-Mar-2016;7:31
How to cite this URL: Granata F, Morabito R, Grasso G, Alafaci E, Salpietro FM, Alafaci C. A rare case of solitary brain Langerhans cell histiocytosis with intratumoral hemorrhage in a patient affected by Turner syndrome. Surg Neurol Int 30-Mar-2016;7:31. Available from: http://surgicalneurologyint.com/surgicalint_articles/a-rare-case-of-solitary-brain-langerhans-cell-histiocytosis-with-intratumoral-hemorrhage-in-a-patient-affected-by-turner-syndrome/
Abstract
Background:Langerhans cell histiocytosis (LCH) is a rare disease involving clonal proliferation of cells with characteristics similar to bone marrow-derived Langerhans cells. The case of a young woman, affected by Turner syndrome and a solitary intraparenchymal LCH associated with an osteolytic lesion of the overlying skull, is presented.
Case Description:The patient, with an insidious history of headache and a growing soft mass in the left frontal region, presented with a sudden generalized tonic-clonic epileptic seizure. Neuroradiological investigations showed an osteolytic lesion of the left frontal bone and an underlying brain lesion associated with recent signs of bleeding. The patient was operated on with a complete removal of the lesion. The postoperative course was uneventful.
Conclusions:The clinical, neuroradiological, and intraoperative findings are presented, along with a review of the literature. Although rare, LCH should be considered in the differential diagnosis when a scalp lesion occurs with a progressive growing.
Keywords: Hemorrhage, Langerhans cell histiocytosis, skull neoplasm
INTRODUCTION
Histiocytic disorders are a group of diseases derived from macrophages and dendritic cells resulting in a wide range of clinical conditions.[
We describe the case of a young female, affected by Turner syndrome (TS), with a solitary brain LCH associated with an osteolytic lesion of the overlaying skull extending to the surrounding subgaleal soft tissues.
CASE REPORT
Clinical presentation
A 30-year-old woman affected by TS, suffering from headache, vomiting, and increase in body temperature was admitted. Her past medical history was characterized by myoclonic seizures, pharmacologically treated, since she was 8-year-old. Four months before the admission, she noticed a progressive growing nodular lesion in the left frontal side of the skull, which was painful on palpation. An ultrasound examination revealed an extracranial isoechoic mass diagnosed as a dermoid cyst.
At admission, the patient developed a generalized tonic-clonic epileptic seizure. She underwent a computed tomography (CT) examination which showed an osteolytic lesion, 33 mm in diameter, of the left frontal bone extending to the surrounding subgaleal soft tissues. The lytic lesion had the “punched-out” appearance, caused by the asymmetric destruction of the bone. There was no evidence of periosteal reaction or bone sclerosis. CT scan also demonstrated an irregular parenchymal left frontal mass, below the bone defect. The tumor had an inhomogeneous density, with some hyperdense components, expressing a recent intratumoral bleeding. Thoraco-abdominal CT scan did not demonstrate abnormal findings in other organs, including lungs, pancreas, and spleen.
Magnetic resonance (MR) examination was performed. The lesion showed strong and irregular increased signal intensity on T1-weighted images and mixed low-high intensity on T2-weighted images. The presence of low- and high-signal components suggested different phases of intratumoral hemorrhage. After gadolinium administration, a slight peripheral enhancement of the lesion was demonstrated.
Treatment
The patient underwent surgical treatment. Following the left frontal skin incision, the bone defect was detected. A surrounding craniotomy was performed. The dura appeared regular without macroscopic signs of tumor infiltration. The lesion, which macroscopically appeared soft and reddish blue with bleeding signs, was completely removed. A cranioplasty, by using methyl methacrylate, was performed to reconstruct the bone defect. The postoperative course was uneventful. Postoperative MR imaging revealed no residual tumor.
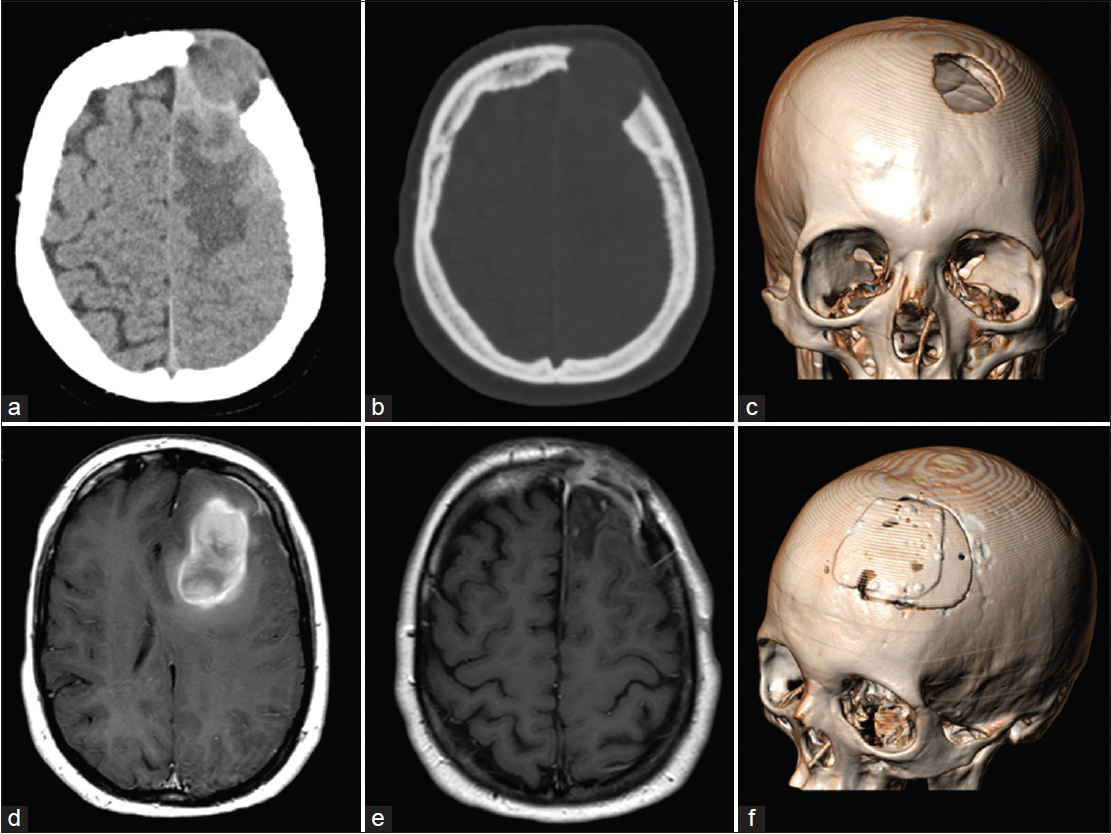
Figure 1
(a and b) Computed tomography scan showing an osteolytic lesion of the left frontal bone, extending to the surrounding subgaleal soft tissues. An irregular left frontal lobe mass, below the bone defect, was also present. (c) Three-dimensional computed tomography scan showing the frontal bone defect. (d) Magnetic resonance examination showing the lesion with an irregular increased signal intensity on T1-weighted images and the coexistence of low- and high-signal components, suggesting different phases of intratumoral hemorrhage. After gadolinium administration, a slight peripheral enhancement was evident. (e) Postoperative magnetic resonance imaging revealing the contrast enhancement of the dural layer with no residual tumor. (f) Three-dimensional computed tomography scan showing the reconstruction of the bone defect
Histological examination showed a normal dura. The brain lesion showed numerous blood cells and deposits of hemosiderin. The tumor was made up of eosinophils, lymphocytes, macrophage with hemosiderin, and many histiocytes in addition to many Langerhans giant cells. The histiocytes and Langerhans cells were positive for S-100 and CD1a proteins. The histological diagnosis was LCH.
DISCUSSION
LCH is a rare immunologic disorder involving clonal proliferation and accumulation in multiple organs of histiocyte-like cells (Langerhans cell) deriving from bone marrow and capable of migrating from skin to lymph nodes.[
LCH has an estimated incidence of 3–5 pediatric cases per million/year and 1–2 adult cases per million/year.[
The disease is confined in one organ in about 55% of the cases whereas in the remaining cases, it presents as a multisystem disease.[
The multifocal unisystem is approximately 20% of LCH cases and usually involves 2–5-year-old children.[
The multifocal multisystem is approximately the 10% of LCH. It is typically diagnosed in the first 2 years of life and it is characterized by a poor prognosis due to the involvement of the reticulo-endothelial system, anemia, thrombocytopenia, and respiratory distress.[
Pathogenetic mechanisms underlying the LCH is a matter of debate. One of the hypotheses is that LCH can be an immunological disorder characterized by an overexpression and/or deficient cytokine activation control of cell proliferation and differentiation into immunocompetent or hematopoietic lines.[
Cases of familiar LCH have rarely been described.[
A relationship between the diffuse form of Langerhans histiocytosis, acute lymphoblastic leukemia, and malignant lymphomas has also been reported.[
We have reported the case of a young patient with a solitary brain LCH with intratumoral bleeding associated with an osteolytic lesion of the skull. Intracranial LCH with intratumoral hemorrhage is a rare condition. According to the literature, only one case of a parietal LCH lesion associated with bleeding components has been reported.[
In our case, LCH was found in the skull, with the characteristic of a well-defined lytic lesion in the brain. Since the dura layer was not infiltrated, we can provide evidence of a double LCH localization. Furthermore, our patient was also affected by TS. TS is a congenital condition that affects 1/2500 births, resulting from the absence or structural alteration of the second sex chromosome. It is usually associated with short stature, gonadal dysgenesis, and variable dysmorphic features.[
CONCLUSIONS
We report a very rare occurrence of a double LCH involving the skull and the overlaying brain, sparing the dura.
Although rare, LCH affecting the brain should be considered, especially when a scalp lesion with a progressive growing occurs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alexiou GA, Mpairamidis E, Sfakianos G, Prodromou N. Cranial unifocal Langerhans cell histiocytosis in children. J Pediatr Surg. 2009. 44: 571-4
2. Bergmann M, Yuan Y, Brück W, Palm KV, Rohkamm R. Solitary Langerhans cell histiocytosis lesion of the parieto-occipital lobe: A case report and review of the literature. Clin Neurol Neurosurg. 1997. 99: 50-5
3. Cai S, Zhang S, Liu X, Lin Y, Wu C, Chen Y. Solitary Langerhans cell histiocytosis of frontal lobe: A case report and literature review. Chin J Cancer Res. 2014. 26: 211-4
4. Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992. 360: 258-61
5. Cho DY, Liau WR, Chiang IP. Eosinophilic granuloma with acute epidural hematoma: A case report. Pediatr Neurosurg. 2001. 35: 266-9
6. Ghafoori S, Mohseni S, Larijani B, Mohajeri-Tehrani MR. Pituitary stalk thickening in a case of langerhans cell histiocytosis. Arch Iran Med. 2015. 18: 193-5
7. Haltrich I, Pikó H, Pamjav H, Somogyi A, Völgyi A, David D. Complex X chromosome rearrangement associated with multiorgan autoimmunity. Mol Cytogenet. 2015. 8: 51-
8. Hoover KB, Rosenthal DI, Mankin H. Langerhans cell histiocytosis. Skeletal Radiol. 2007. 36: 95-104
9. Lajolo C, Campisi G, Deli G, Littarru C, Guiglia R, Giuliani M. Langerhans's cell histiocytosis in old subjects: Two rare case reports and review of the literature. Gerodontology. 2012. 29: e1207-14
10. Larizza D, Calcaterra V, Martinetti M. Autoimmune stigmata in Turner syndrome: When lacks an X chromosome. J Autoimmun. 2009. 33: 25-30
11. Lee BD, Lee W, Lee J, Son HJ. Eosinophilic granuloma in the anterior mandible mimicking radicular cyst. Imaging Sci Dent. 2013. 43: 117-22
12. Lee BH, George S, Kutok JL. Langerhans cell histiocytosis involving the thymus. A case report and review of the literature. Arch Pathol Lab Med. 2003. 127: e294-7
13. Martínez-Lage JF, Bermúdez M, Martínez-Barba E, Fuster JL, Poza M. Epidural hematoma from a cranial eosinophilic granuloma. Childs Nerv Syst. 2002. 18: 74-6
14. Moscinski LC, Kleinschmidt-DeMasters BK. Primary eosinophilic granuloma of frontal lobe. Diagnostic use of S-100 protein. Cancer. 1985. 56: 284-8
15. Mosiewicz A, Rola R, Jarosz B, Trojanowska A, Trojanowski T. Langerhans cell histiocytosis of the parietal bone with epidural and extracranial expansion - Case report and a review of the literature. Neurol Neurochir Pol. 2010. 44: 196-203
16. Nicollas R, Rome A, Belaïch H, Roman S, Volk M, Gentet JC. Head and neck manifestation and prognosis of Langerhans’ cell histiocytosis in children. Int J Pediatr Otorhinolaryngol. 2010. 74: 669-73
17. Saliba I, Sidani K, El Fata F, Arcand P, Quintal MC, Abela A. Langerhans’ cell histiocytosis of the temporal bone in children. Int J Pediatr Otorhinolaryngol. 2008. 72: 775-86
18. Uranishi R, Nikaido Y, Eguchi T, Bessho H, Fujimoto T, Inui T. Eosinophilic granuloma associated with intratumoral hemorrhage – Case report. Neurol Med Chir (Tokyo). 1996. 36: 458-61
19. Watanabe S, Yamamoto T, Satomi K, Matsuda M, Akutsu H, Ishikawa E. Comparison of magnetic resonance imaging with invasive histological findings of Langerhans cell histiocytosis. Brain Tumor Pathol. 2014. 31: 182-6
20. Zaveri J, La Q, Yarmish G, Neuman J. More than just Langerhans cell histiocytosis: A radiologic review of histiocytic disorders. Radiographics. 2014. 34: 2008-24