- Department of Neurosurgery, Johns Hopkins Medical Institutes, Baltimore, Maryland, USA
- Department of Radiation Oncology and Molecular Radiation Sciences, Johns Hopkins Medical Institutes, Baltimore, Maryland, USA
- Department of Oncology, Johns Hopkins Medical Institutes, Baltimore, Maryland, USA
Correspondence Address:
Michael Lim
Department of Neurosurgery, Johns Hopkins Medical Institutes, Baltimore, Maryland, USA
Department of Radiation Oncology and Molecular Radiation Sciences, Johns Hopkins Medical Institutes, Baltimore, Maryland, USA
Department of Oncology, Johns Hopkins Medical Institutes, Baltimore, Maryland, USA
DOI:10.4103/sni.sni_140_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Yuanxuan Xia, Leila A. Mashouf, Russell Maxwell, Luke C. Peng, Evan J. Lipson, William H. Sharfman, Chetan Bettegowda, Kristin J. Redmond, Lawrence R. Kleinberg, Michael Lim. Adjuvant radiotherapy and outcomes of presumed hemorrhagic melanoma brain metastases without malignant cells. 26-Jul-2018;9:146
How to cite this URL: Yuanxuan Xia, Leila A. Mashouf, Russell Maxwell, Luke C. Peng, Evan J. Lipson, William H. Sharfman, Chetan Bettegowda, Kristin J. Redmond, Lawrence R. Kleinberg, Michael Lim. Adjuvant radiotherapy and outcomes of presumed hemorrhagic melanoma brain metastases without malignant cells. 26-Jul-2018;9:146. Available from: http://surgicalneurologyint.com/surgicalint-articles/adjuvant-radiotherapy-and-outcomes-of-presumed-hemorrhagic-melanoma-brain-metastases-without-malignant-cells/
Abstract
Background:Patients with melanoma can present with a hemorrhagic intracranial lesion. Upon resection, pathology reports may not detect any malignant cells. However, the hemorrhage may obscure their presence and so physicians may still decide whether adjuvant radiotherapy should be applied. Here, we report on the outcomes of a series of patients with melanoma with hemorrhagic brain lesions that returned with no tumor cells.
Methods:All melanoma patients who had craniotomies from 2008 to 2017 at a single institution for hemorrhagic brain lesions were identified through retrospective chart review. Those who had pathology reports with no malignant cells were analyzed. Recurrence at the former site of hemorrhage and resection was the primary outcome.
Results:Ten patients met inclusion criteria, and the median follow-up time was 8.5 (1.8–27.3) months. At the time of craniotomy, the median number of brain lesions was 3 (1–25). Two patients had prior craniotomies, eight had prior radiation, and six had prior immunotherapy to the lesion of interest. After surgery, one patient received stereotactic radiosurgery (SRS) to the resection bed. Only one patient developed subsequent melanoma at the resection site; this patient developed the lesion recurrence once and had not received postoperative SRS.
Conclusion:Although small foci of metastatic disease as a source of bleeding for some patients cannot be excluded, melanoma patients with a suspected hemorrhagic brain metastasis that shows no tumor cells on pathology may benefit from close observation. The local recurrence risk in such cases appears to be low, even without adjuvant radiation.
Keywords: Brain metastasis, hemorrhage, melanoma, negative pathology, stereotactic radiosurgery
INTRODUCTION
Worldwide, the incidence of brain metastases is increasing in patients with advanced solid tumors and this is in part due to better systemic therapies and increased surveillance with improved imaging modalities.[
When a hemorrhagic brain lesion is discovered in a patient with melanoma, it is commonly presumed to be a melanoma metastasis. Typically, standard of care involves surgical resection and, once pathology confirms melanoma, a regimen of stereotactic radiosurgery (SRS) is prescribed to significantly reduce the risk of local recurrence.[
In an effort to minimize unnecessary side effects, only close interval observation is often used when no tumor is found after resection. However, there is a risk for misdiagnosis in hemorrhagic lesions since their bloody composition may dilute out malignant cells. Moreover, the necrotic features of melanoma have been known to make it difficult to diagnose on pathology.[
MATERIALS AND METHODS
This retrospective series received institutional review board approval. Medical records were reviewed for all patients with melanoma who underwent craniotomy by a senior author between 2008 and 2017. Inclusion criteria for relevant cases were patients (1) over 18 years of age, (2) with a history of melanoma, (3) presenting with hemorrhagic intracranial lesions, and (4) with surgical specimens reporting no tumor by pathological examination. Data on patient demographics (age at diagnosis, age at relevant surgical resection, sex), characteristics of melanoma disease (BRAF mutation status, extent of metastasis), intracranial disease course (age at relevant brain lesion diagnosis, total number, and location of brain lesions), previous treatments, presenting symptoms, surgical parameters (relevant lesion and size, pathology report, extent of resection), treatment after relevant surgery, and outcomes of interest were collected from electronic medical records. Relevant pathology reports were categorized as either radiation necrosis if “treatment effect” was recorded or gliosis and inflammatory infiltrate if such findings were recorded in the pathology reports. The primary outcome measure was pathology-confirmed melanoma at the site of resection that previously reported no tumor. Secondary outcome measures included other metastatic intracranial sites before last date of follow-up. Systemic treatments before or after the relevant surgery were considered to affect the lesion of interest. All analyses were performed in STATA SE 14 (StataCorp, College Station, TX, USA) and statistical significance was defined as P ≤ 0.05.
RESULTS
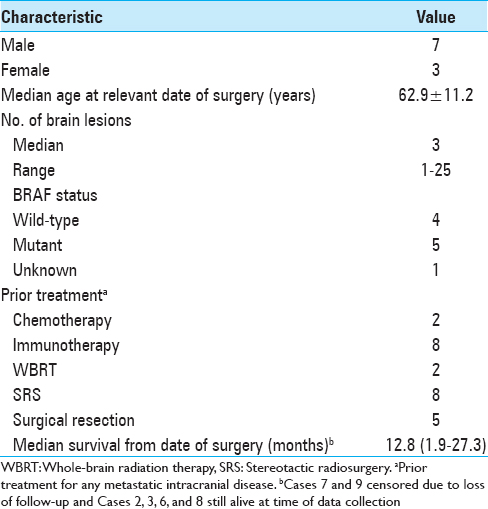
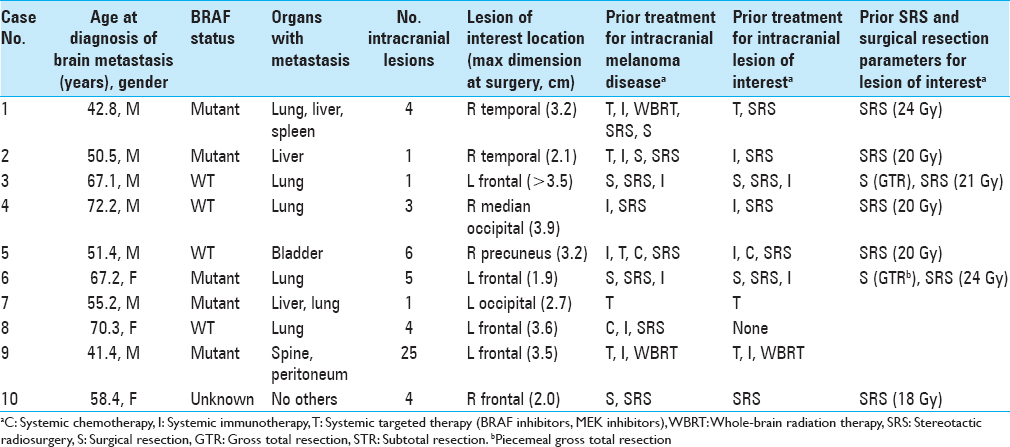
During the study period, 69 separate craniotomy cases were performed to resect 77 lesions in patients with melanoma. Among them, 10 cases (13.9%) of individual patients met the inclusion criteria. All cases that produced surgical specimens reporting no tumor were also hemorrhagic lesions. Two of these patients had de novo lesions that quickly hemorrhaged while the other eight had hemorrhages in lesions that were already documented. The median age at the date of surgery was 62.9 years. Patient demographic information is summarized in
Illustrative cases
Case 4 – Local tumor development after a lesion with ambiguous pathology
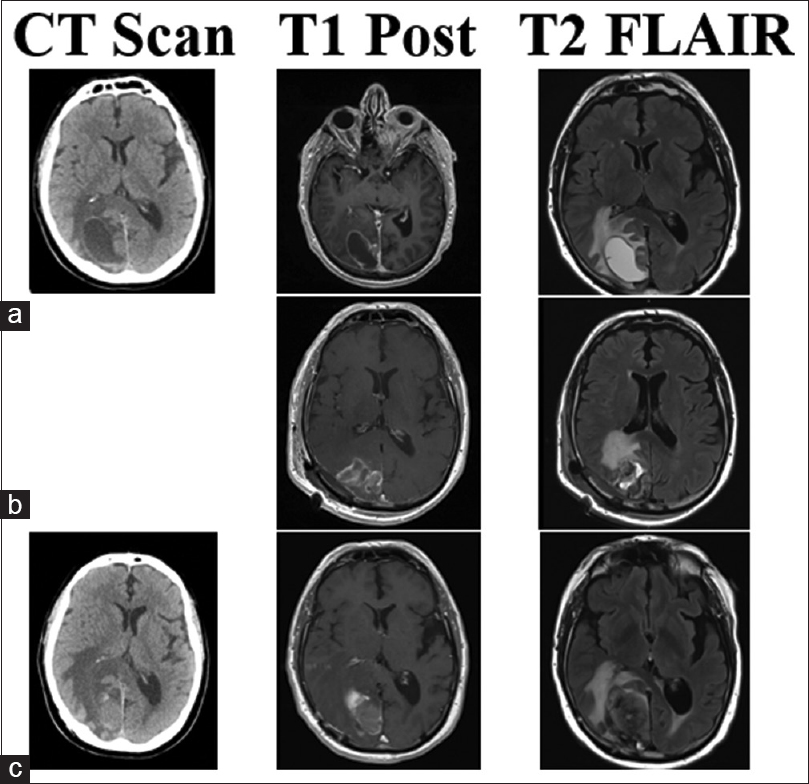
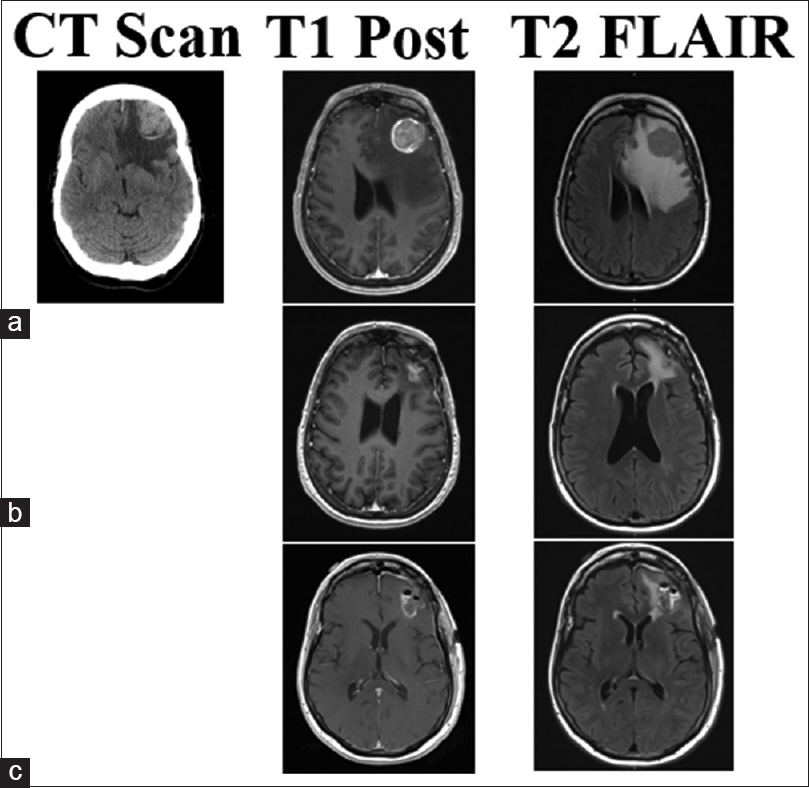
This 70-year-old male patient had two separate primary cutaneous diagnoses: one lesion in the left upper abdomen and another on the upper back. Both lesions were wild-type BRAF. Over the following 18 months, the patient developed multiple systemic cutaneous lesions that were resected. Two years after his primary diagnosis, a lesion was discovered in his left cingulate gyrus. SRS was applied to this lesion and the patient was given immunotherapy with ipilimumab. Despite treatment, the patient's systemic disease progressed and alternative immunotherapy was initiated with combined nivolumab and lirilumab 16 months after brain metastases were found. Four and 8 months later, lesions in the right postcentral gyrus and right medial occipital lobe were found, respectively, and SRS was promptly applied to both (20 Gy each). The right medial occipital lesion hemorrhaged shortly after SRS but was managed conservatively for 8 months until the patient struck his head during a fall. His lesion subsequently expanded, became symptomatic, and required surgical intervention. Neuroimaging identified a 2.3-cm lesion with a 3.9 cm surrounding cystic area [
Figure 1
CT, T1 post-contrast, and T2 Flair images for illustrative Case 4. This patient's lesion in the right occipital lobe was treated with SRS (20 Gy) less than 1 month before hemorrhage with nivolumab and lirilumab immunotherapy. (a) Imaging of the hemorrhagic lesion that produced samples with chronic inflammation and no viable tumor cells. Post-operative MRI shows gross total resection in (b), no CT was performed at the time. The lesion recurred and hemorrhaged in 4 months as seen in (c). The second surgery produced samples with melanoma tumor cells and confirmed the lesion as metastasis
Case 6 – No local tumor development after a lesion with ambiguous pathology
This 63-year-old female patient had a primary diagnosis of BRAF-positive cutaneous melanoma, although the specific lesion that became melanotic was unclear due to the patient's extensive history of multiple compound nevi and skin lesions. Over the following 4 years, the patient had local surgical resection of several cutaneous lesions. Metastases developed in the patient's lungs and brain and neuroimaging first detected three separate lesions in the left frontal, right deep parieto-occipital, and left occipital lobes 4 years after the patient's primary diagnosis. These lesions progressed, and the patient's left frontal lobe lesion eventually caused cognitive deficits that required surgical intervention [
Figure 2
CT, T1 post-contrast, and T2 Flair images for illustrative Case 6. This patient's lesion of interest in the left frontal lobe progressed (a) and required surgery, which produced samples confirming melanoma metastasis. Postoperative SRS (24 Gy) was given with systemic nivolumab immunotherapy. However, the lesion recurred and hemorrhaged in 18 months (b), requiring a second resection with postoperative imaging shown in (c). No CTs were performed. This second surgery produced samples of gliosis, inflammation, and no tumor cells so no SRS was applied. There has been no local recurrence to date
DISCUSSION
Melanoma brain metastases hemorrhage more frequently than all other types of primary and secondary brain tumors.[
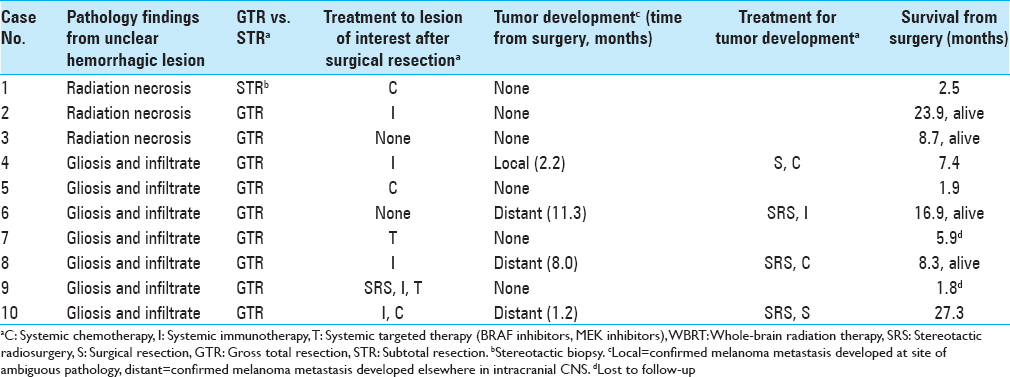
In this series of 10 melanoma patients, 3 patients (Cases 1–3) had a hemorrhagic lesion consistent with radiation necrosis while 7 (Cases 4–10) had ambiguous findings of gliosis and inflammatory infiltrate. Only one lesion (Case 4), originally noted as gliosis and inflammatory infiltrate, had a local recurrence that proved to be melanoma. In cases of true metastatic melanoma to the brain, adjuvant radiotherapy, usually in the form of SRS, is administered to increase both the rates of intracranial disease control and patient survival time.[
In the one case of this series with local recurrence, the preoperative therapies that were given may have influenced the hemorrhage that occurred. Recently, data have suggested that immunotherapy combined with SRS increases overall survival and is associated with higher complication rates including hemorrhage and radiation necrosis.[
The bloody nature of hemorrhagic lesions can simultaneously make pathologic diagnoses more challenging and also reduce local recurrence risks by facilitating better resections. Tumor cells that are actually in a lesion may be missed since hemorrhage may obscure or dilute them from samples. In Illustrative Case 4, the prolonged 8-month period of non-surgical management after the lesion of interest had hemorrhaged may have complicated detection of any actual tumor cells present. Indeed, brain lesions in the setting of systemic cancer are not necessarily treated surgically. Surgical resection is often reserved for symptomatic or large lesions while asymptomatic or small lesions may be observed or treated with only radiotherapy.[
This case series should be considered exploratory: due to the rare nature of this phenomenon, this study encompasses a small sample size with experience from only one institution. Further studies with larger sample sizes and prospective designs are warranted to better define the risk of melanoma recurrence in a suspected hemorrhagic metastasis with no tumor cells on pathologic examination. In addition, a subsequent local recurrence that proves to be melanotic does not necessarily imply that tumor cells were always present but missed during the initial resection. Rather, the local recurrence of true melanoma could be independent of the original lesion, since patients with melanoma have ongoing systemic disease that can generate new metastases. Nonetheless, the aim of this study was to evaluate the risk of local recurrence and whether adjuvant radiotherapy can still be beneficial. This case series shows that hemorrhagic lesions do not seem to have frequent local recurrences that are truly melanotic.
CONCLUSION
In this series, it appears that melanoma patients who present with a suspected hemorrhagic brain metastasis that instead shows no viable tumor cells may be managed with close observation rather than immediate adjuvant radiation. Here, only one patient experienced a local lesion recurrence that proved to be melanoma. Clinicians should be aware of this low risk of recurrence as the incidence of melanoma continues to rise worldwide.
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All procedures met ethical standards of the institution at which this retrospective study was conducted and informed consent was obtained from the individual participants described in Illustrative Cases 4 and 6.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
EJL is a consultant for Bristol-Myers Squibb and receives research grants from Bristol-Myers Squibb and Merck. WHS is a consultant for Merck and receives research grants from Bristol-Myers Squibb, Merck, and Novartis. KJR receives research grants and travel compensation from Elekta AB and Accuray and receives honoraria from AstraZeneca (educational activities) and Accuray. LRK receives research grants from and serves on the advisory board for Novocure and receives research grants, travel compensation, and honoraria from Accuray. ML serves on the advisory board of Merck, Agenus, Oncorus, Boston Biomedical, and Baxter; receives research grants from Agenus and Immunocellular; is a consultant for Stryker; and serves on the Speaker's Bureau for Stryker. All other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
This article does not contain information about medical device(s)/drug(s). No funds were received in support of this work.
References
1. Alomari AK, Cohen J, Vortmeyer AO, Chiang A, Gettinger S, Goldberg S. Possible interaction of anti-PD-1 therapy with the effects of radiosurgery on brain metastases. Cancer Immunol Res. 2016. 4: 481-7
2. Bekaert L, Emery E, Levallet G, Lechapt-Zalcman E. Histopathologic diagnosis of brain metastases: Current trends in management and future considerations. Brain Tumor Pathol. 2017. 34: 8-19
3. Berghoff AS, Preusser M. Targeted therapies for melanoma brain metastases. Curr Treat Options Neurol. 2017. 19: 13-
4. Bindal RK, Sawaya R, Leavens ME, Hess KR, Taylor SH. Reoperation for recurrent metastatic brain tumors. J Neurosurg. 1995. 83: 600-4
5. Braeuer RR, Watson IR, Wu C-J, Mobley AK, Kamiya T, Shoshan E. Why is melanoma so metastatic?. Pigment Cell Melanoma Res. 2014. 27: 19-36
6. Cohen J V, Tawbi H, Margolin KA, Amravadi R, Bosenberg M, Brastianos PK. Melanoma central nervous system metastases: Current approaches, challenges, and opportunities. Pigment Cell Melanoma Res. 2016. 29: 627-42
7. Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases?. J Neurosurg. 2016. 125: 17-23
8. Fonkem E, Uhlmann EJ, Floyd SR, Mahadevan A, Kasper E, Eton O. Melanoma brain metastasis: Overview of current management and emerging targeted therapies. Expert Rev Neurother. 2012. 12: 1207-15
9. Frinton E, Tong D, Tan J, Read G, Kumar V, Kennedy S. Metastatic melanoma: Prognostic factors and survival in patients with brain metastases. J Neurooncol. 2017. 135: 507-12
10. Gans JH, Raper DMS, Shah AH, Bregy A, Heros D, Lally BE. The role of radiosurgery to the tumor bed after resection of brain metastases. Neurosurgery. 2012. 72: 317-26
11. Glitza Oliva I, Tawbi H, Davies MA. Melanoma brain metastases: Current areas of investigation and future directions. Cancer J. 2017. 23: 68-74
12. Halasz LM, Rockhill JK. Stereotactic radiosurgery and stereotactic radiotherapy for brain metastases. Surg Neurol Int. 2013. 4: S185-
13. Harris KB, Corbett MR, Mascarenhas H, Lee KS, Arastu H, Leinweber C. A Single- institution analysis of 126 patients treated with stereotactic radiosurgery for brain metastases. Front Oncol. 2017. 7: 90-
14. Hatiboglu MA, Wildrick DM, Sawaya R. The role of surgical resection in patients with brain metastases. Ecancermedicalscience. 2013. 7: 308-
15. Iwama T, Ohkuma A, Miwa Y, Sugimoto S, Itoh T, Takada M. Brain tumors manifesting as intracranial hemorrhage. Neurol Med Chir (Tokyo). 1992. 32: 130-5
16. Johnson MD, Baschnagel AM, Chen PY, Krauss DJ, Olson RE, Meyer KD. Analysis of risk factors for development of radiation necrosis following gamma knife radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2013. 87: S279-80
17. Jung S, Moon K-S, Jung T-Y, Kim I-Y, Lee Y-H, Rhu H-H. Possible pathophysiological role of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in metastatic brain tumor-associated intracerebral hemorrhage. J Neurooncol. 2006. 76: 257-63
18. Kaidar-Person O, Zagar TM, Deal A, Moschos SJ, Ewend MG, Sasaki-Adams D. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases. Anticancer Drugs. 2017. 28: 669-75
19. Kondziolka D, Bernstein M, Resch L, Tator CH, Fleming JFR, Vanderlinden RG. Significance of hemorrhage into brain tumors: Clinicopathological study. J Neurosurg. 1987. 67: 852-7
20. Lee LM, Feun L, Tan Y. A case of intracranial hemorrhage caused by combined dabrafenib and trametinib therapy for metastatic melanoma. Am J Case Rep. 2014. 15: 441-3
21. Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012. 13: 459-65
22. Miyatake S-I, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo). 2015. 55: 50-9
23. Nicholas S, Mathios D, Jackson C, Lim M. Metastatic melanoma to the brain: Surgery and radiation is still the standard of care. Curr Treat Options Oncol. 2013. 14: 264-79
24. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ.editors. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998. 280: 1485-9
25. Pekmezci M, Perry A. Neuropathology of brain metastases. Surg Neurol Int. 2013. 4: S245-55
26. Prieto VG, Shea CR. Immunohistochemistry of melanocytic proliferations. Arch Pathol Lab Med. 2011. 135: 853-9
27. Stokes WA, Binder DC, Jones BL, Oweida AJ, Liu AK, Rusthoven CG. Impact of immunotherapy among patients with melanoma brain metastases managed with radiotherapy. J Neuroimmunol. 2017. 313: 118-22
28. Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012. 32: 4655-62
29. Telera S, Fabi A, Pace A, Vidiri A, Anelli V, Carapella CM. Radionecrosis induced by stereotactic radiosurgery of brain metastases: Results of surgery and outcome of disease. J Neurooncol. 2013. 113: 313-25
30. Westphal D, Glitza Oliva IC, Niessner H. Molecular insights into melanoma brain metastases. Cancer. 2017. 123: 2163-75
31. Yoo H, Jung E, Gwak HS, Shin SH, Lee SH. Surgical outcomes of hemorrhagic metastatic brain tumors. Cancer Res Treat. 2011. 43: 102-7