- Department of Neurosurgery, Yala Hospital, Yala, Thailand,
- Department of Neurosurgery, Arkansas Neuroscience Institute, Arkansas, United States,
- Department of Neurosurgery, ISSSTE “1ro de Octubre”, Mexico City, Mexico.
Correspondence Address:
Thitikan Wangapakul, Department of Neurosurgery, Yala Hospital, Yala, Thailand.
DOI:10.25259/SNI_130_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Thitikan Wangapakul1, Abdel Raouf Kayssi2, Ambar Elizabeth Moguel Riley3. Akinetic mutism following bilateral parasagittal meningioma occupied supplementary motor area removal and the spontaneous recovery of symptoms. 03-May-2024;15:150
How to cite this URL: Thitikan Wangapakul1, Abdel Raouf Kayssi2, Ambar Elizabeth Moguel Riley3. Akinetic mutism following bilateral parasagittal meningioma occupied supplementary motor area removal and the spontaneous recovery of symptoms. 03-May-2024;15:150. Available from: https://surgicalneurologyint.com/surgicalint-articles/12880/
Abstract
Background: Resection of bilateral parasagittal meningiomas of the dominant cortex is challenging. Some postoperative consequences are difficult to predict due to their low incidence. However, it is essential to recognize reversible symptoms. Akinetic mutism is a devastating but reversible symptom that occurs after supplementary motor area (SMA) injury. This report aims to provide more information to support the clinical progression of this syndrome.
Case Description: A 47-year-old woman presented with psychomotor retardation and subtle weakness, particularly on the left side. A palpable mass was identified at the head vertex. Magnetic resonance imaging revealed bilateral parasagittal meningiomas with bone and sinus invasion of the SMA. A craniotomy was performed to remove the intracapsular tumor. Two days after the operation, the patient developed gradual deterioration in her motor function until it became a lock-in-like syndrome. Then, 1.5 months after treatment in the hospital and rehabilitation unit, she gradually improved her motor, cognitive, and psychomotor skills. Total recovery was achieved after 1 year.
Conclusion: Surgery for lesions involving bilateral SMA can cause akinetic mutism. The typical manifestation of this syndrome may be devastating. However, it is reversible, and patients can regain full motor and cognitive functions over time without specific treatments. It is crucial to persevere and continue to provide the best care to the patient until recovery.
Keywords: Akinetic mutism, Craniotomy, Magnetic resonance imaging, Parasagittal meningioma, Supplementary motor area
INTRODUCTION
The removal of parasagittal meningioma poses challenges for neurosurgeons, with complication rates potentially reaching as high as 23%.[
The supplementary motor area (SMA), part of Broadmann area 6, is the eloquent area located on the posterior 1/3 of the superior frontal gyrus, the medial surface anterior to the premotor cortex. It is thought to be responsible for initiating movements. Some connections of the SMA to the subthalamic nucleus are presumed to be a hyperdirect pathway that suppresses thalamic circuits, resulting in movement arrest. SMA syndrome is a group of symptoms that occur after SMA function is altered.[
Meningiomas occupying bilateral SMA have become troublesome. The risk of major venous injury and the awareness of SMA damage are critical factors. Total resection is considered impossible due to concerns regarding cortical disturbances. However, injuries are unavoidable. Due to this rare entity, many neurosurgeons are unfamiliar with its symptoms. This report describes the syndrome of akinetic mutism following bilateral parasagittal meningioma in the SMA after resection and how symptoms progress afterwards.
CASE PRESENTATION
Clinical presentation
A 47-year-old woman presented with psychomotor retardation and subtle weakness, particularly on the left side. Her speech was comprehensible but slow. No cranial nerve palsy was observed, and pupillary responses were preserved. She did not complain of headache but had an abnormal palpable, growing deformity in the frontal bone.
Diagnostic imaging
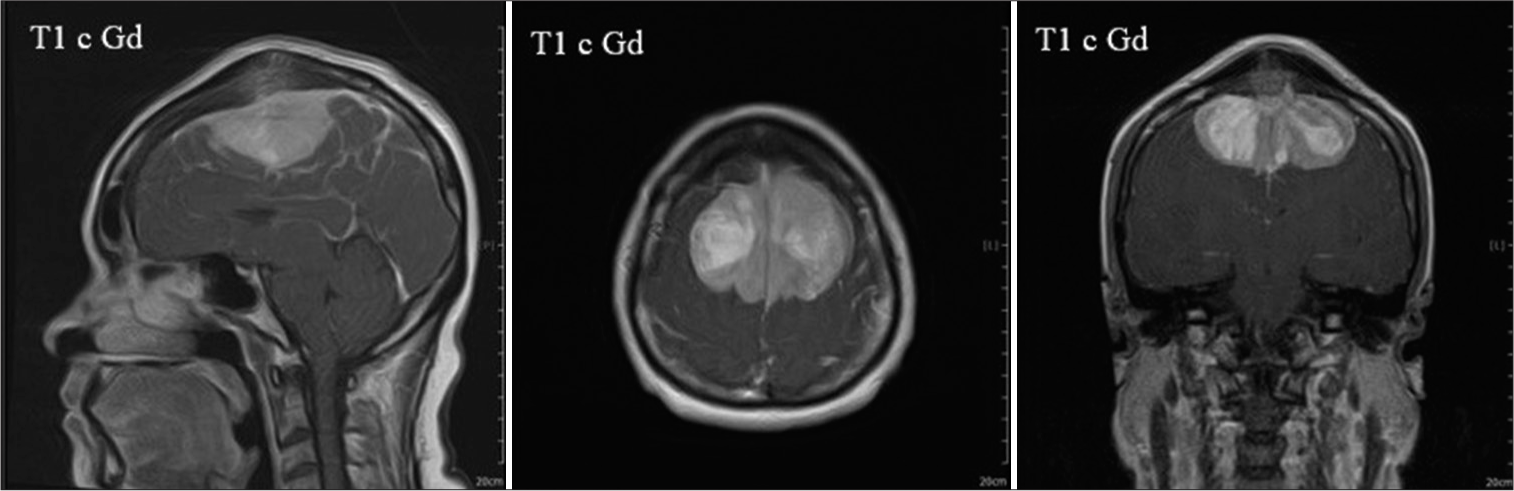
Magnetic resonance imaging (MRI) revealed a bilateral parasagittal meningioma invading the SMA. Bone hyperostosis with the signal intensity of the tumor beneath the scalp was shown. The overall extension area of the tumor involved both SMA cortices, completely occluded the middle 1/3 of the superior sagittal sinus area, and burst into the covered bone [
Operative procedure
A craniotomy was performed to achieve near-total tumor removal. Initially, the tumor was identified as invading the outer cortex of the bone and integrating with the dura mater. A doughnut-shaped craniotomy was performed. The island of bone with the midline tumor was carefully drilled and removed. The tumor completely penetrated the sagittal sinus at the point where it originated, and no sinus wall was identified in the tumor. No venous bleeding was observed from the incision. Internal debulking was also performed, eliminating all tumor masses in the area, including those within the invaded sagittal sinus. The left-only tumor plugged the sagittal sinuses anterior and posterior to the lesion, which was still intact, and a thin layer of the capsule was left in place to avoid eloquent cortex injury. A pericranium graft was used, and skin closure was performed. We did not perform cranioplasty immediately after tumor removal because we considered the patient to be at high risk of postoperative cerebral edema.
Clinical progression
The patient was conscious and could follow instructions during an immediate postoperative evaluation. Motor strength decreased in the right leg, but the patient could still raise it against gravity. Conversely, the strength of all other extremities was intact. Extubation was performed after 24 hours of observation. However, the patient’s motor response slowed several hours later, although she could still communicate. Motor function progressively deteriorated until she could not raise her extremities. The following day, the patient’s motor function was completely unresponsive to external stimuli. Her eyes blinked and moved spontaneously in response to voices and commands. There were no movements of any limbs or head. The patient was reintubated to prevent airway collapse and sent for computed tomography (CT) to evaluate a possible infarction or other complications. No large infarcts are other than a moderate degree of perioperative edema were observed. A brain MRI was done a day after the patient’s clinical condition became stable, and no infarction showed in diffusion-weighted imaging. A follow-up CT did not show any hemorrhage. Pathological study revealed a meningothelial meningioma (the World Health Organization grade I).
She stayed in the intensive care unit for 1 month and had spontaneous eye-opening on voice calls or light. This process seemed static until the end of the month, when her eye responses became more frequent, and some motor twitching was observed. After 1.5 months, she was transferred back for rehabilitation at a local hospital. The first recovery was observed in the right and left legs.
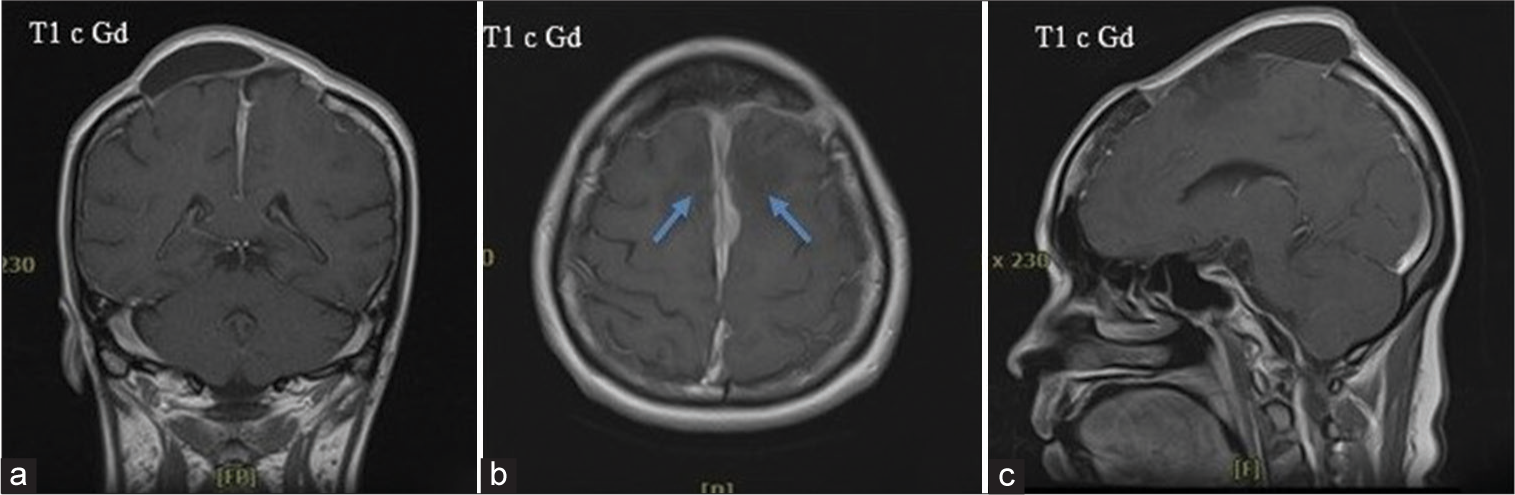
The recovery process accelerated afterwards. The upper extremity function returned. In a couple of weeks, the patient progressed to ambulation, recognized more people, and began communicating faster 2.5 months after the operation. She visited a clinic and was able to walk in with slight difficulty. Her response was faster than the preoperative status. Speech fluency improved, and psychomotor retardation noticeably disappeared. After all symptoms progression and clinical improvement, the patient was diagnosed with akinetic mutism. Follow-up MRI was done at 3 months, showing minimal residual along the falx cerebri [
DISCUSSION
Akinetic mutism is caused by pathologies in several areas of the brain, especially the mesencephalic-diencephalic region, cingulate gyrus, mesial frontal lobe, and, more often, the SMA.[
Several authors have described the clinical presentation and progression of symptoms after surgery.
The exact cause of this phenomenon can be attributed to many pathologies that affect this area. In this case, SMA injury might be caused by venous insufficiency, which, in this case, we noticed the occlusion of the main sagittal sinus from the preoperative investigation. However, the development of the surrounding cortical venous drainage system maintained adequate circulation before surgery. Another possible cause is tissue damage by mechanical forces or heat used during tumor dissection. This may have temporarily caused the eloquent area to become inflamed and edematous. After the pathology subsided, brain tissue recovered. These symptoms are reversible and motor, sensory, or cognitive.[
There are several learning points in the present case. First, while preservation of the capsule to avoid injury to the cortex is a prudent strategy, injury still occurs due to thermal damage. Therefore, it is crucial to avoid using monopolar cautery and decrease bipolar power to the minimum effective level. Second, the cortical venous system may be essential in developing postoperative venous infarction. The collateral cortical veins drain cerebral blood circulation even when the sagittal sinus becomes occluded. Care should be taken to avoid injury to these cortical veins, particularly when the brain environment changes.
CONCLUSION
This report aimed to share data supporting the idea that akinetic mutism, which may occur after bilateral SMA meningioma resection, is a reversible phenomenon. Most patients fully recover without any neuro deficits after months. Therefore, it is critical to recognize this symptom.
Proper postoperative care should be provided to the patient regardless of consciousness level, and relevant complications, such as deep vein thrombosis or joint stiffness, should be prevented. Adjunct supportive procedures, such as tracheostomy, should be performed to avoid the risk of pneumonia, and when the clinical symptoms subside, the tracheostomy tube can be removed afterwards.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We thank the patient for allowing us to share her vital information. We also thank coworkers and teachers who encouraged us.
References
1. Ackermann H, Ziegler W. Akinetischer mutismus--eine literaturübersicht [Akinetic mutism--a review of the literature]. Fortschr Neurol Psychiatr. 1995. 63: 59-67
2. Arnts H, van Erp WS, Lavrijsen JC, van Gaal S, Groenewegen HJ, van den Munckhof P. On the pathophysiology and treatment of akinetic mutism. Neurosci Biobehav Rev. 2020. 112: 270-8
3. Bederson JB, Eisenberg MB. Resection and replacement of the superior sagittal sinus for treatment of a parasagittal meningioma: Technical case report. Neurosurgery. 1995. 37: 1015-8 discussion 1018-9
4. Berg-Johnsen J, Høgestøl EA. Supplementary motor area syndrome after surgery for parasagittal meningiomas. Acta Neurochir (Wien). 2018. 160: 583-7
5. Borghei-Razavi H, Raghavan A, Eguiluz-Melendez A, Joshi K, Fernandez-Miranda JC, Kshettry VR. Anatomical variations in the location of veins draining into the anterior superior sagittal sinus: Implications for the transbasal approach. Oper Neurosurg. 2020. 18: 668-75
6. Cañas A, Juncadella M, Lau R, Gabarrós A, Hernández M. Working memory deficits after lesions involving the supplementary motor area. Front Psychol. 2018. 9: 765
7. Chen WW, Wang Y, Hu YC, Zhao YL. Analysis of the common complications and recurrence-related factors of superior parasagittal sinus meningioma. Front Surg. 2022. 9: 1023021
8. Heiferman DM, Ackerman PD, Hayward DM, Primeau MJ, Anderson DE, Prabhu VC. Bilateral supplementary motor area syndrome causing akinetic mutism following parasagittal meningioma resection. Neurosci Discov. 2014. 2: 7
9. Ma J, Song T, Hu W, Muhumuza ME, Zhao W, Yang S. Reconstruction of the superior sagittal sinus with silicone tubing. Neurosurg Focus. 2002. 12: ecp1
10. Martínez-Pérez R, Vergara C, Rayo N, Mura J. Recurrent supplementary motor area syndrome in relapsed parasagittal meningiomas: From the onset to the origin. Neurologia (Engl Ed). 2020. 35: 606-8
11. Nowak A, Dziedzic T, Czernicki T, Kunert P, Marchel A. Surgical treatment of parasagittal and falcine meningiomas invading the superior sagittal sinus. Neurol Neurochir Pol. 2014. 48: 174-80
12. Palmisciano P, Haider AS, Balasubramanian K, Dadario NB, Robertson FC, Silverstein JW. Supplementary motor area syndrome after brain tumor surgery: A systematic review. World Neurosurg. 2022. 165: 160-71.e2
13. Pinson H, Van Lerbeirghe J, Vanhauwaert D, Van Damme O, Hallaert G, Kalala JP. The supplementary motor area syndrome: A neurosurgical review. Neurosurg Rev. 2022. 45: 81-90
14. Satter AR, Asif DS, Zannat S, Gaddam SK. Postoperative supplementary motor area syndrome: A case report. Mymensingh Med J. 2017. 26: 451-4
15. Shamov T, Al-Hashel J, Rousseff RT. Postoperative supplementary motor area syndrome: Clinical evolution and prognosis in nine patients after left hemispheric tumor resection. Hippokratia. 2020. 24: 38-42
16. Sindou MP, Alvernia JE. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major Dural sinuses. J Neurosurg. 2006. 105: 514-25
17. Sjöberg RL, Stålnacke M, Andersson M, Eriksson J. The supplementary motor area syndrome and cognitive control. Neuropsychologia. 2019. 129: 141-5
18. Tsai CC, Su YF, Tsai FJ, Su HY, Ko HJ, Cheng YH. Supplementary motor area syndrome after removal of an unusual extensive parasagittal meningioma: Analysis of twelve reported cases. Medicina. 2022. 58: 1126