- Department of Neurosurgery, Royal Prince Alfred Hospital, 50 Missenden Road, Camperdown, New South Wales 2050, Australia
- Department of Neurosurgery, St Vincent's Hospital, 390 Victoria Street, Darlinghurst, New South Wales 2010, Australia
- Faculty of Medicine, University of Sydney, Camperdown, New South Wales 2050, Australia
- Department of Nuclear Medicine and Theranostics, St Vincent's Hospital, 390 Victoria Street, Darlinghurst, New South Wales 2010, Australia
Correspondence Address:
Charles Fish
Department of Nuclear Medicine and Theranostics, St Vincent's Hospital, 390 Victoria Street, Darlinghurst, New South Wales 2010, Australia
DOI:10.4103/sni.sni_189_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Charles Fish, David Wilson, Biyi Chen, Charlotte Yin. Cerebral abscesses after endovascular coiling of a paraophthalmic aneurysm: Case report and review of the literature. 13-Dec-2018;9:252
How to cite this URL: Charles Fish, David Wilson, Biyi Chen, Charlotte Yin. Cerebral abscesses after endovascular coiling of a paraophthalmic aneurysm: Case report and review of the literature. 13-Dec-2018;9:252. Available from: http://surgicalneurologyint.com/surgicalint-articles/9139/
Abstract
Background:Intracranial infections are a rare complication of therapeutic neuroendovascular procedures.
Case Description:We present a case of a 72-year-old female with multiple unilateral cerebral hemisphere abscesses after endovascular embolization of a right paraophthalmic aneurysm and also provide a comprehensive review of the literature on cerebral abscesses following neurovascular embolization.
Conclusion:Infection following coil embolization of cerebral aneurysm is rare. However, it is likely to increase in the setting of increased used of neuroendovascular techniques in the future. Therefore, we suggest that extreme care is taken to ensure proper asepsis during embolization, and a high index of suspicion is maintained in patients with predisposing characteristics (large hemorrhage, ischemia, recurrent endovascular procedures, right-to-left shunt, and concomitant infection). Given the fact that the majority of abscesses occurred in patients who have had ruptured aneurysms, we suggest consideration is given to prophylactic intraprocedural intravenous antibiotics use as seen with open aneurysm treatment.
Keywords: Abscesses, aneurysm, complication, neuroendovascular
INTRODUCTION
Endovascular embolization is an important therapeutic option for cerebral aneurysms and cerebral arteriovenous malformations (AVMs), and provide an alternative to surgical clipping; especially, for patients who are poor surgical candidates.[
CASE REPORT
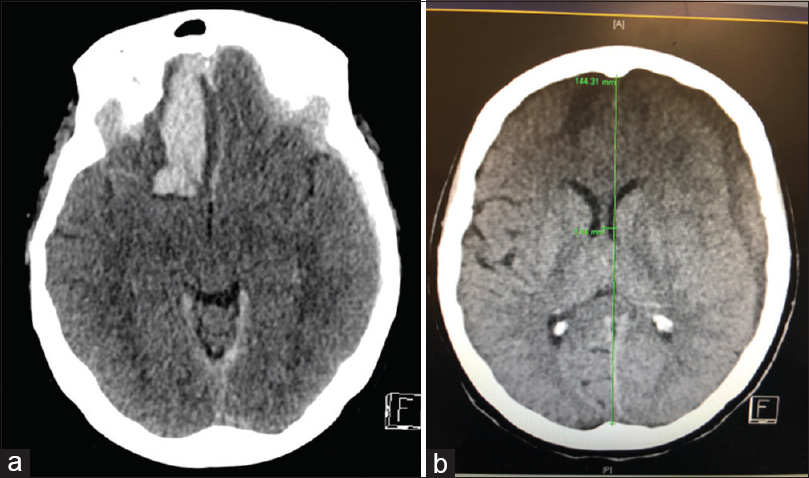
A 72-year-old female presented with a Fisher Grade 3 and World Federation of Neurological Societies Grade V aneurysmal subarachnoid hemorrhage (SAH) and an acute left traumatic subdural hematoma (SDH) post fall [Figure
Figure 2
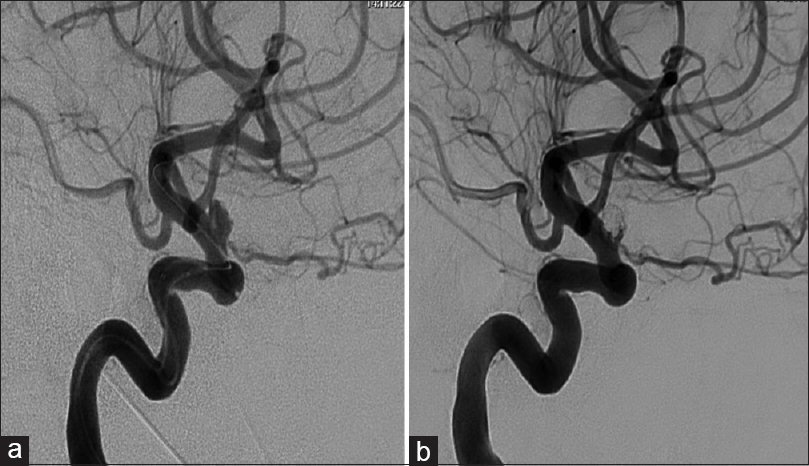
(a) Angiography (anteroposterior view) confirms the presence of a 5.5 × 3.4 × 3.7 mm superiorly projecting aneurysm arising from the supraclinoid internal carotid artery (ICA) with a 2.5 mm neck. (b) Angiography showing good occlusion of the fundus but some residual neck filling of the right ICA
She underwent balloon assisted coiling of the right paraophthalmic aneurysm using microvention coils one day after the SAH [
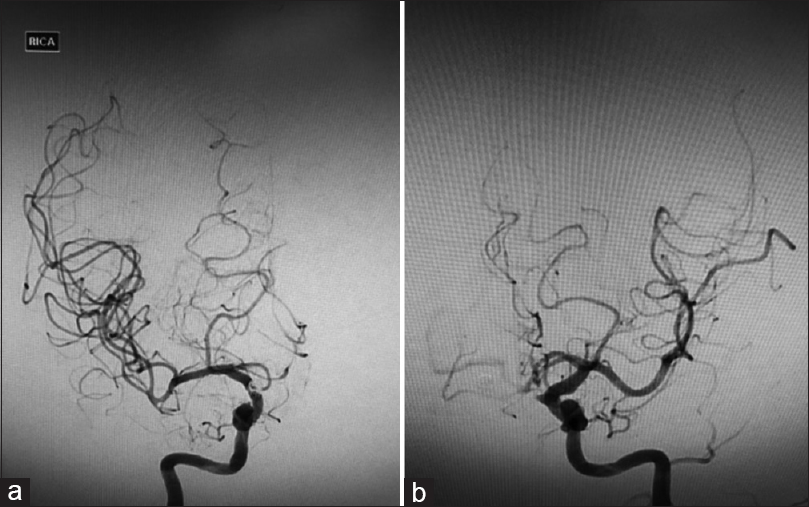
Angiography one week post coiling showed severe vasospasm of the bilateral A1s and its distal branches [Figure
Three weeks after the intra-arterial treatment, the patient became hemodynamically unstable and developed a right sided hemiparesis. CT scan showed evolution of the left acute SDH into a chronic SDH with mass effect. She underwent an uneventful burr hole evacuation of the left SDH with resolution of her right-sided hemiparesis.
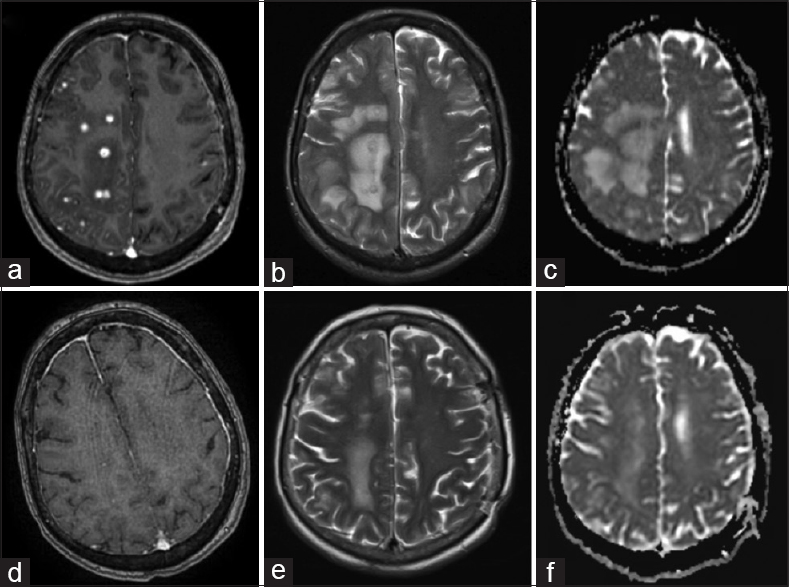
Her post-treatment course was complicated by vasospasm requiring two take backs for endovascular intra-arterial treatment and evacuation of the left SDH. CT one month after admission showed a new 27×25 mm2 low density area in the right posterior frontal lobe suggestive of ischemia. She remained a febrile with no signs of meningism, however, developed worsening left-sided hemiparesis and confusion. Magnetic resonance imaging (MRI) showed significant enhancement around the coil mass and multiple ring-enhancing lesions in the right frontal, parietal, and occipital lobes with surrounding edema [Figure
Figure 4
(a) T1-weighted (T1W) axial MRI with gad shows multiple ring-enhancing abscesses in the right frontal, parietal, and occipital lobes with surrounding edema. (b) Corresponding T2-weighted (T2W) axial image showed multiple hyperintense centers and hypointense irregular rims. (c) The abscesses appear isointense with areas of hypointensity on apparent diffusion coefficient (ADC) map suggestive of restricted diffusion. One month after the commencement of antimicrobial therapy, MRI demonstrated resolution in many of the enhancing foci with significant decrease in the size of the surrounding vasogenic edema (e-f)
Intra-arterial blood distal to the coils was obtained for culture. Attempted biopsy of the emboli was abandoned after post sampling angiography showed a filling defect at the right middle cerebral artery (MCA) bifurcation. Repeat right ICA angiography showed clearance at the MCA bifurcation with migration of the thrombus to a single M2/M3 branch. Thrombectomy was not attempted because of the risks of hemorrhage in the setting of possible infective arteritis. Peripheral and intra-arterial blood cultures were negative. White cell count WCC, C-reactive protein (CRP), and chest X-Ray (CXR) were unremarkable. Transesophageal echocardiogram was negative for valvular vegetations. She was commenced on dexamethasone and intravenous vancomycin 1g BD, ceftriaxone 2g BD, and fluconazole 400 mg. Tissue biopsy was not taken in light of antibiotic commencement and the likelihood of obtaining a sterile sample.
One month after the commencement of antimicrobial therapy, MRI brain demonstrated resolution of many of the enhancing foci with significant decrease in the size of the surrounding vasogenic edema [Figure
DISCUSSION
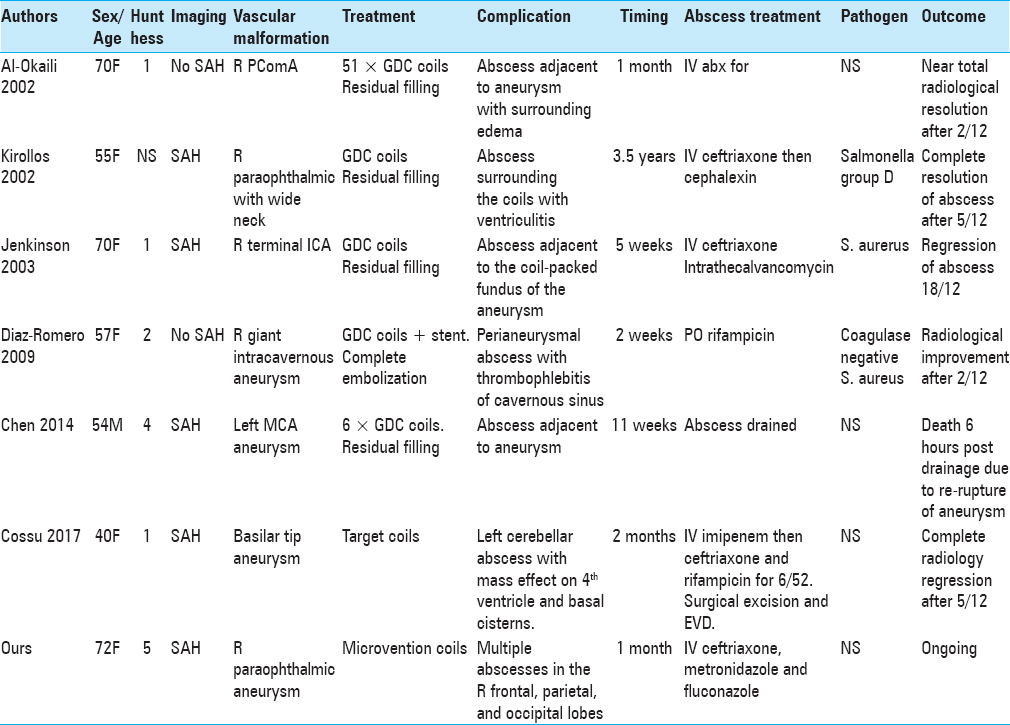
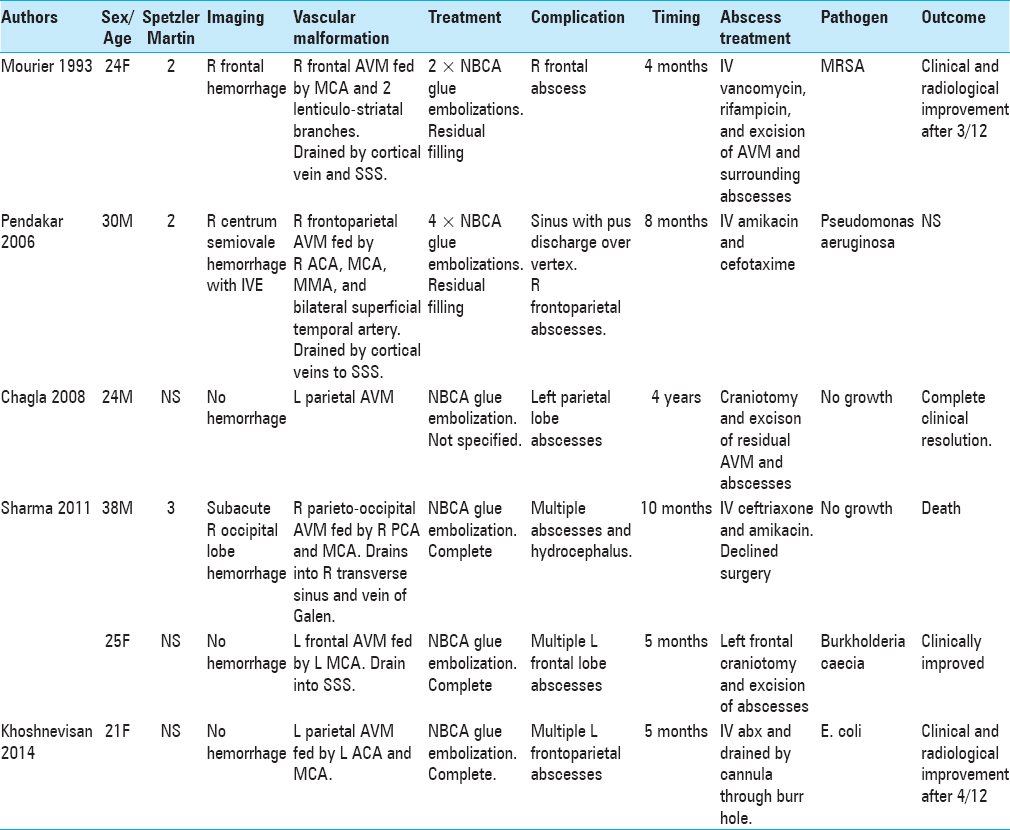
A search was conducted using MEDLINE and PubMed of all English language case reports published between January 1945 and January 2018, which describe intracranial abscesses post embolization. The following search terms were used in combination: “cerebral,” “abscess,” “endovascular,” and “embolization.” Review of literature found 12 patients (13 including our case) satisfying these criteria.[
A review of literature found that cerebral abscess in the context of endovascular procedures occurs irrespective of the age, gender, location of aneurysm or AVM, and the clinical and radiological grade of the SAH [Tables
There were 9 females and 4 males. Their mean age at diagnosis was 40 ± 18 years (range 21–72 years). In seven patients,[
Six females[
Three males[
All 13 patients had CT or MRI scans that showed a single abscess in 6 patients (46.2%) and multiple cerebral abscesses in 7 patients (53.8%). Singular abscesses were common in aneurysm patients post endovascular treatment, whereas multiple abscesses were more common in AVM patients. Abscesses are more likely to develop adjacent to aneurysms than AVMs and may be explained by the majority of aneurysm patients in the literature having ruptured aneurysms on initial presentation. All abscesses were located in the same hemisphere as the aneurysm or AVM.
Pathogens were identified in 7 out of 13 patients. In 3 out of 13 patients (2 aneurysms, 1 AVM), Staphyloccous aureus was present in blood culture.[
Kelkar et al.'s retrospective review of 2918 cerebral angiograms and neurointerventional procedures performed without prophylactic antibiotics found that the risk of infection attributable to angiography was 0.1% with all infections that were found localized to the femoral artery puncture site with no systemic and no CNS complications.[
The majority of patients (92.3%) were treated with antimicrobial therapy for at least 4 weeks. A total of 8 patients (3 aneurysm and 5 AVM, 53.8%) also underwent surgical treatment: 6 surgical drainages (1 aneurysm, 5 AVM), 1 surgical drainage plus insertion of external ventricular drain (EVD), 1 insertion of EVD that was then changed to a ventriculoperitoneal shunt for hydrocephalus, and 3 surgical excisions of AVMs. The only patient who did not receive 4 weeks of antimicrobial therapy passed away shortly after drainage of his abscess due to aneurysm re-rupture.[
A total of 10 out of 13 patients had a favorable outcome (including ours to date) with clinical and radiological regression or resolution of the abscess/s. A total of 2 patients had an unfavorable outcome: one died shortly after surgical drainage of the abscess due to aneurysm re-rupture and another with AVM died after declining surgery.
Cerebral abscess results from predisposing factors such as disruption of the blood-brain barrier, underlying disease, or a systemic source of infection (e.g., endocarditis or bacteremia).[
Disruption of the blood-brain barrier allows the infectious agent to penetrate the intimal wall and cause an intraparenchymal infection. Three key causes include hemorrhage, edema, and ischemia. The cerebral inflammatory response is initiated at the time of aneurysmal rupture with erythrocytes that accumulate in the subarachnoid space lysing and releasing inflammatory cytokines.[
In the normal brain, the blood-brain barrier provides resistance to infection but its disruption during ischemia and hemorrhage opens the way to abscess growth. With a high incidence of bacteremia during therapeutic angiography, the risk of infection is heightened if the procedure causes any ischemia.[
Cerebral abscesses following therapeutic endovascular procedures are a rare but an important complication associated with significant morbidity and mortality. The incidence of CNS infections is only expected to become more prevalent with the increased use of endovascular procedures.
In our patient, a polyvinylpyrrolidone (PVP) infection is likely to have been introduced during the endovascular procedure or during intra-arterial vasospasm treatments using the sheath, catheters, or balloon. This is supported by the 4-week interval between the procedure and the formation of the abscesses on the same hemisphere as the coils. In addition, our patient had no other systemic infection or congenital abnormality such as a right-to-left shunt that could have led to hematogenous dissemination. The infectious agents could have penetrated the aneurysm wall and caused intraparenchymal infection when the integrity of the blood-brain barrier is disrupted by hemorrhage and ischemia as is likely to have occurred in our patient.
Our patient also had poor prognostic factors (Grade V hemorrhage and older age) that likely triggered a strong cerebral inflammatory response that was responsible for the surrounding edema and periods of ischemia from the lack of blood supply around the inert coils after an efficient embolization and then prolonged periods of vasospasms during multiple intra-arterial treatments.
CONCLUSION
PVP encephalitis following coil embolization of cerebral aneurysm is rare and more likely to occur in the presence of hemorrhage, edema, and ischemia. Therefore, we suggest that extreme care is taken to ensure proper asepsis during embolization, and a high index of suspicion is maintained in patients with predisposing characteristics (large hemorrhage, ischemia, recurrent endovascular procedures, right-to-left shunt, and concomitant infection). Given 5 out of 7 SAH patients reported were ruptured, we suggest consideration is given to prophylactic intraprocedural IV antibiotics use as seen with open aneurysm treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. AL-Okaili R, Patel SJ. Brain abscess after endovascular coiling of a saccular aneurysm: Case Report. Am J Neuroradiol. 2002. 23: 697-9
2. Brouwer MC, Tunkel AR, McKhann GMI, van de Beek D. Brain abscess. N Engl JMed. 2014. 371: 447-56
3. Chagla AS, Balasubramaniam S. Cerebral N-butyl cyanoacrylate glue-induced abscess complicating embolization. J Neurosurg. 2008. 109: 347-
4. Chen G, Zhan S, Chen W, Li Z, Zhou D, Zeng S. Brain abscess after endosaccular embolisation of a cerebral aneurysm. J Clin Neurosci. 2014. 21: 163-5
5. Chen S-T, Tang L-M, Ro L-S. Brain abscess as a complication of stroke. Stroke. 1995. 26: 696-8
6. Cossu G, Daniel RT, Messerer M. Cerebral abscess after neuro-vascular embolization: Own experience and review of the literature. Acta Neurochir (Wien). 2017. 159: 583-91
7. Diaz-Romero R, Zenteno M, Santos-Franco JA, Soto-Hernandez JL, Lee A. Perianeurysmal abscess and meningitis after endovascular coil placement for an intracranial aneurysm. SurgInfect (Larchmt). 2009. 10: 359-62
8. Emmez H, Borcek AO, Dogulu F, Ceviker N. Ischemic stroke complicated by a brain abscess: A case report and review of the literature. TurkNeurosurg. 2007. 17: 48-54
9. Falagas ME, Nikou SA, Siempos II. Infections related to coils used for embolization of arteries: Review of the published evidence. J Vasc Interv Radiol. 2007. 18: 697-701
10. Hanafy KA, Morgan Stuart R, Fernandez L, Schmidt JM, Claassen J, Lee K. Cerebral inflammatory response and predictors of admission clinical grade after aneurysmal subarachnoid hemorrhage. JClin Neurosci. 2010. 17: 22-5
11. Jenkinson MD, Javadpour M, Nixon T, Warnke P. Intracerebral abscess formation following embolisation of an internal carotid artery aneurysm using Guglielmi detachable coils. Acta Neurochir (Wien). 2003. 145: 703-6
12. Kelkar PS, Fleming JB, Walters BC, Harrigan MR. Infection risk in neurointervention and cerebral angiography. Neurosurgery. 2013. 72: 327-31
13. Khoshnevisan A, Ghorbani A, Sistany Allahabadi N, Farzaneh F, Abdollahzadeh S, Soleymani S. Cerebral abscess complicating embolization of an arteriovenous malformation: Case report and review of litera. Iran J Neurol. 2014. 13: 181-4
14. Kim H-K, Hwang S-K, Kim S-H. Types of thromboembolic complications in coil embolization for intracerebral aneurysms and management. J Korean Neurosurg Soc. 2009. 46: 226-31
15. Kirollos RW, Bosma JJ, Radhakrishnan J, Pigott TD. Endovascularly treated cerebral aneurysm using Guglielmi detachable coils acting as a nidus for brain abscess formation secondary to Salmonella bacteremia: Case report. Neurosurgery. 2002. 51: 234-7
16. Lucke-Wold BP, Logsdon AF, Manoranjan B, Turner RC, McConnell E, Vates GE. Aneurysmal subarachnoid hemorrhage and neuroinflammation: A comprehensive review. Int JMol Sci. 2016. 17: 497-
17. Mourier KL, Bellec C, Lot G, Reizine D, Gelbert F, Dematons C. Pyogenic parenchymatous and nidus infection after embolization of an arteriovenous malformation. An unusual complication. Case report Acta Neurochir. 1993. 122: 130-3
18. Pendarkar H, Krishnamoorthy T, Purkayastha S, Gupta AK. Pyogenic cerebral abscess with discharging sinus complicating an embolized arteriovenous malformation. J Neuroradiol. 2006. 33: 133-8
19. Seibert B, Tummala RP, Chow R, Faridar A, Mousavi SA, Divani AA. Intracranial aneurysms: Review of current treatment options and outcomes. Front Neurol. 2011. 2: 45-
20. Sharma A, Jagetia A, Loomba P, Singh D, Tandon M. Delayed brain abscess after embolization of arterio-venous malformation: Report of two cases and review of literature. Neurol India. 2011. 59: 620-23
21. Tummala RP, Chu RM, Madison MT, Myers M, Tubman D, Nussbaum ES. Outcomes after aneurysm rupture during endovascular coil embolization. Neurosurgery. 2001. 49: 1059-66