- Professor of Clinical Neurosurgery, School of Medicine, State University of New York at Stony Brook, Mineola, New York, USA
- Chief of Neurosurgical Spine and Education, Winthrop NeuroScience, NYU Winthrop Hospital, Mineola, New York, USA
Correspondence Address:
Nancy E. Epstein
Professor of Clinical Neurosurgery, School of Medicine, State University of New York at Stony Brook, Mineola, New York, USA
Chief of Neurosurgical Spine and Education, Winthrop NeuroScience, NYU Winthrop Hospital, Mineola, New York, USA
DOI:10.4103/sni.sni_433_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. Cerebrospinal fluid drains reduce risk of spinal cord injury for thoracic/thoracoabdominal aneurysm surgery: A review. 23-Feb-2018;9:48
How to cite this URL: Nancy E. Epstein. Cerebrospinal fluid drains reduce risk of spinal cord injury for thoracic/thoracoabdominal aneurysm surgery: A review. 23-Feb-2018;9:48. Available from: http://surgicalneurologyint.com/surgicalint-articles/cerebrospinal-fluid-drains-reduce-risk-of-spinal-cord-injury-for-thoracic-thoracoabdominal-aneurysm-surgery-a-review/
Abstract
Background:The risk of spinal cord injury (SCI) due to decreased cord perfusion following thoracic/thoracoabdominal aneurysm surgery (T/TL-AAA) and thoracic endovascular aneurysm repair (TEVAR) ranges up to 20%. For decades, therefore, many vascular surgeons have utilized cerebrospinal fluid drainage (CSFD) to decrease intraspinal pressure and increase blood flow to the spinal cord, thus reducing the risk of SCI/ischemia.
Methods:Multiple studies previously recommend utilizing CSFD following T/TL-AAA/TEVAR surgery to treat SCI by increasing spinal cord blood flow. Now, however, CSFD (keeping lumbar pressures at 5–12 mmHg) is largely utilized prophylactically/preoperatively to avert SCI along with other modalities; avoiding hypotension (mean arterial pressures >80–90 mmHG), inducing hypothermia, utilizing left heart bypass, and employing intraoperative neural monitoring [somatosensory (SEP) or motor evoked (MEP) potentials]. In addition, preoperative magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) scans identify the artery of Adamkiewicz to determine its location, and when/whether reimplantation/reattachment of this critical artery and or other major segmental/lumbar arterial feeders are warranted.
Results:Utilizing CSFD for 15–72 postoperative hours in T/TL-AAA/TEVAR surgery has reduced the risks of SCI from a maximum of 20% to a minimum of 2.3%. The major complications of CSFD include; spinal and cranial epidural/subdural hematomas, VI nerve palsies, retained catheters, meningitis/infection, and spinal headaches.
Conclusions:By increasing blood flow to the spinal cord during/after T/TL-AAA/TEVAR surgery, CSFD reduces the incidence of permanent SCI from, up to 10-20% down to down to 2.3-10%. Nevertheless, major complications, including spinal/cranial subdural hematomas, still occur.
Keywords: Abdominal aneurysm (T/TL-AAA) surgery, cerebrospinal fluid drainge (CSFD), complications, lumbar, spinal hematomas, subdural hematomas, TEVAR lumbar drain, thoracic
INTRODUCTION
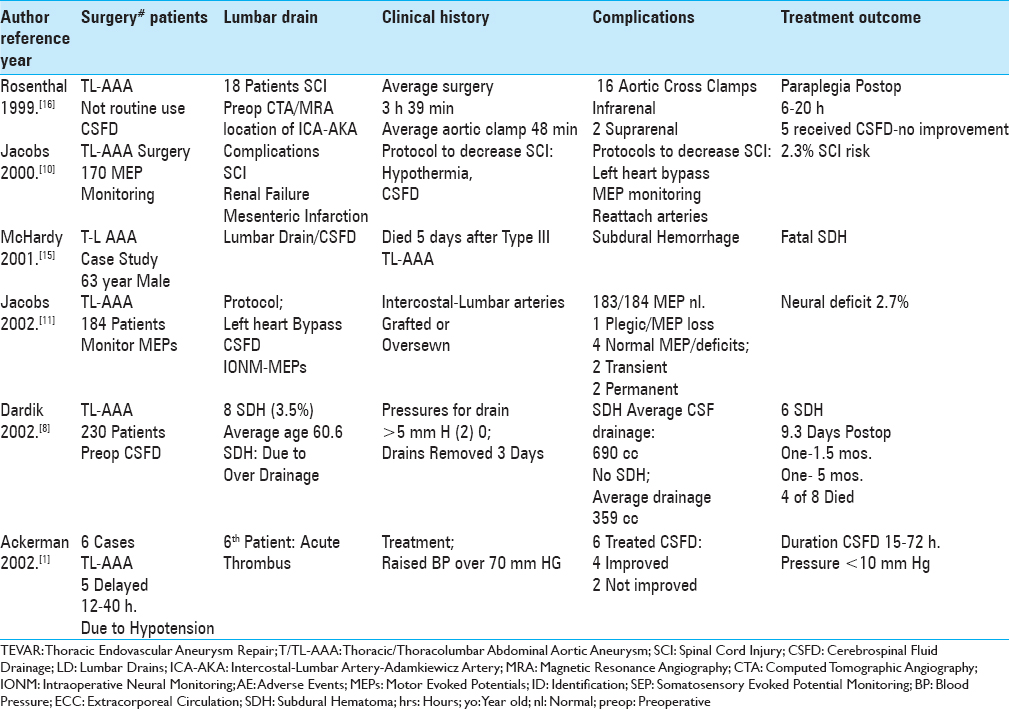
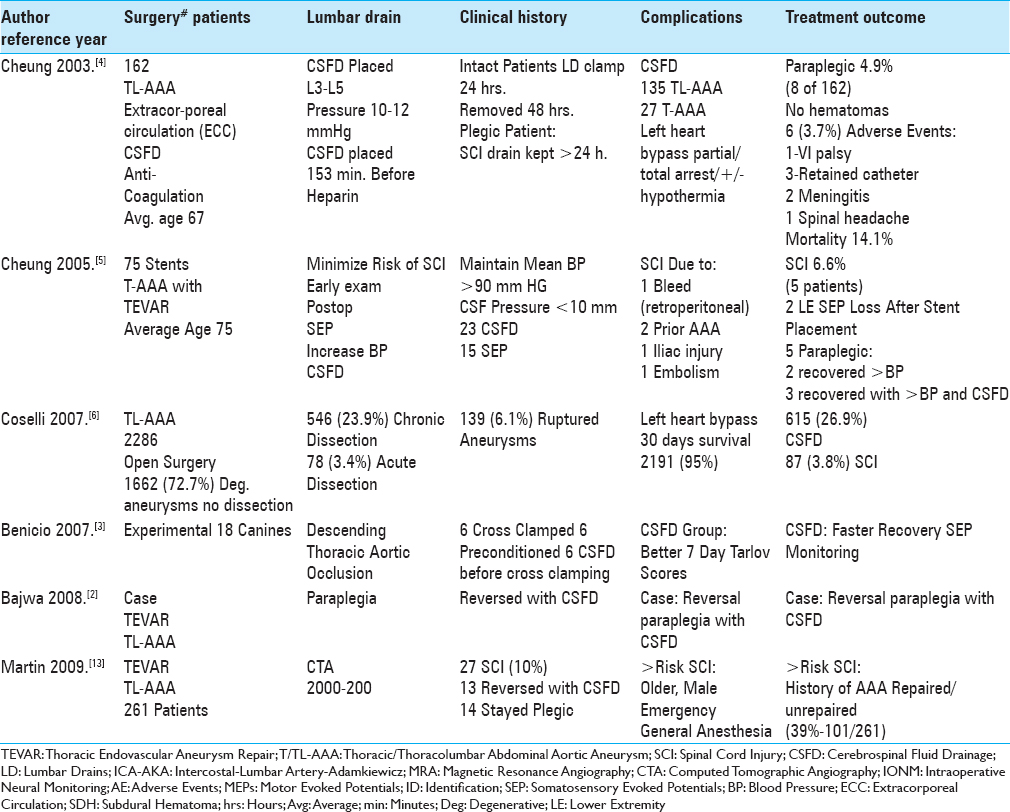
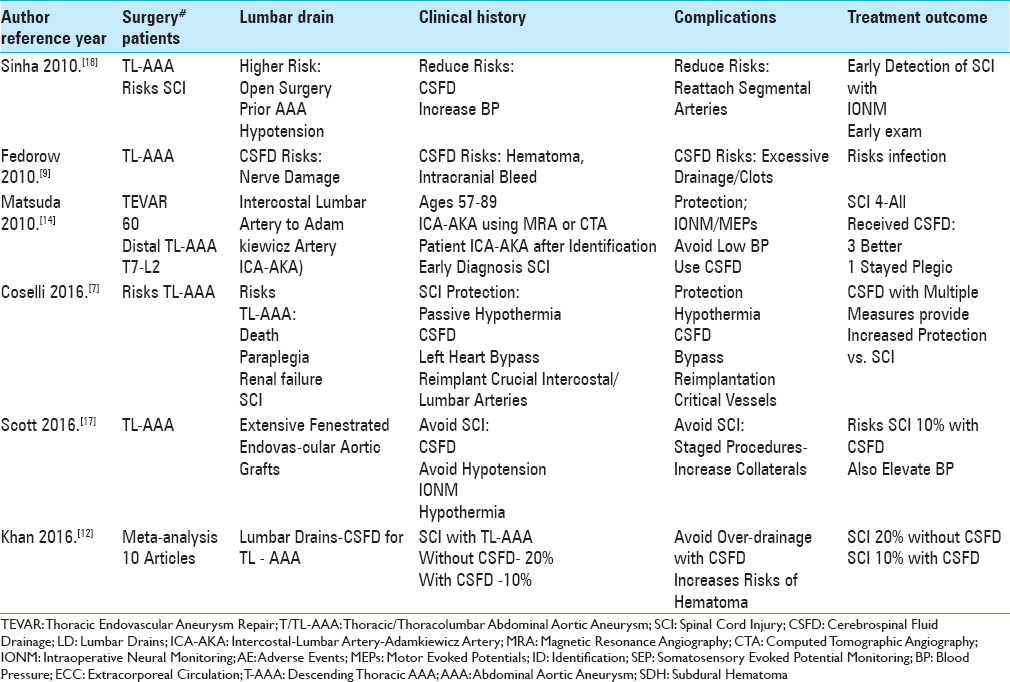
Here we reviewed the pros, cons, and complications of cerebrospinal fluid drains (CSFD) used in thoracic/thoracoabdominal aneurysm surgery (T/TL-AAA)/thoracic endovascular aneurysm repair (TEVAR) [Tables
MATERIALS AND METHODS
We identified 18 articles utilizing PubMed and the terms “Thoracic/Thoracoabdominal Aortic Aneurysm Surgery (T/TL-AAA),” “Thoracic Endovascular Aneurysm Repair (TEVAR),” “Cerebrospinal Fluid Drainage (CSFD),” and “Spinal/Neurological Complications” to assess the pros, cons, risks, and complications without and with CSFD in patients undergoing these procedures [Tables
1986 definition of Crawford types I–IV of TL-AAA
In 1986, Crawford described four types of TL-AAA.[
Canine study; CSFD avoids SCI with experimental thoracic aortic occlusion
To determine the onset of SCI, Benicio et al. cross-clamped the descending thoracic aorta (60 minutes) in 18 canines divided into 3 groups; 6 controls (only aortic cross clamping), 6 with ischemic preconditioning, and 6 with prophylactic CSFD (e.g. lumbar drains opened just before cross clamping) [
Incidence of spinal cord injury/paraplegia with and without CSFD
The risk of spinal cord injury (SCI) without CSFD for TL-AAA ranges up to 20%, while with CSFD it was reduced to 2.3–10% [Tables
Higher incidence of SCI without preoperative CSFD for T/TL-AAA/TEVAR
Several studies documented the higher rates (10-20%) of SCI occurring when CSFD was not employed prior to TL-AAA/TEVAR surgery [Tables
Reduction of SCI with preoperative placement of CSFD for T/TL-AAA/TEVAR
The placement of CSFD prior to T/TL-AAA/TEVAR surgery reduced the postoperative incidence of SCI to 2.3–10% [Tables
Risk factors for SCI with TL-AAA/TEVAR surgery
Several studies cited risks factors for SCI occurring after TL-AAA//TEVAR [Tables
Measures to prevent SCI
Multiple measures utilized to prevent SCI following TL-AAA/TEVAR included; prophylactic placement of CSFD, increasing the mean arterial blood pressure (>80–90 mmHg), mild passive hypothermia, early neurological assessment postoperatively to detect the onset of paraparesis/paraplegia, evaluation of preoperative MRA/CTA to determine the necessity of reimplanting intercostal/lumbar arteries, staging surgical procedures to promote collateral circulation, identifying the vascular supply to the artery of Adamkiewicz, and the use of intraoperative neural monitoring (IONM: SEP/MEP) [Tables
Intraoperative neural monitoring limits paraplegia following TL-AAA surgery
Following TL-AAA/TEVAR surgery, the acute/subacute onset of SCI was typically attributed to reduced spinal cord perfusion (e.g., decreased collateral circulation/perfusion of the artery of Adamkiewicz, hypotension), resulting in increased spinal cord ischemia/edema/reperfusion injury, and increased intraspinal pressures [
Multiple adverse events still result from CSFD utilized in T/TL-AAA/TEVAR surgery
Many adverse events were still attributed to utilizing CSFD to avoid SCI for patients undergoing T/TL-AAA/TEVAR [Tables
Subdural Hematomas (SDH) resulting from CSFD
Nine acute SDH were reported in two studies.[
CONCLUSIONS
Without CSFD, patients undergoing T/TL–AAA/TEVAR sustained up to a 20% risk of SCI, while with CSFD, SCI were reduced to a minimum of 2.3% [Tables
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ackerman LL, Traynelis VC. Treatment of delayed-onset neurological deficit after aortic surgery with lumbar cerebrospinal fluid drainage. Neurosurgery. 2002. 51: 1414-21
2. Bajwa A, Davis M, Moawad M, Taylor PR. Paraplegia following elective endovascular repair of abdominal aortic aneurysm: Reversal with cerebrospinal fluid drainage. Eur J Vasc Endovasc Surg. 2008. 35: 46-8
3. Benício A, Moreira LF, Mônaco BA, Castelli JB, Mingrone LE, Stolf NA. Comparative study between ischemic preconditioning and cerebrospinal fluid drainage as methods of spinal cord protection in dogs. Rev Bras Cir Cardiovasc. 2007. 22: 15-23
4. Cheung AT, Pochettino A, Guvakov DV, Weiss SJ, Shanmugan S, Bavaria JE. Safety of lumbar drains in thoracic aortic operations performed with extracorporeal circulation. Ann Thorac Surg. 2003. 76: 1190-
5. Cheung AT, Pochettino A, McGarvey ML, Appoo JJ, Fairman RM, Carpenter JP. Strategies to manage paraplegia risk after endovascular stent repair of descending thoracic aortic aneurysms. Ann Thorac Surg. 2005. 80: 1280-8
6. Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2007. 83: S862-4
7. Coselli JS, de la Cruz KI2Preventza O, LeMaire SA, Weldon SA. Extent II Thoracoabdominal Aortic Aneurysm Repair: How I Do It. Semin Thorac Cardiovasc Surg. 2016. 28: 221-37
8. Dardik A, Perler BA, Roseborough GS, Williams GM. Subdural hematoma after thoracoabdominal aortic aneurysm repair: An underreported complication of spinal fluid drainage?. J Vasc Surg. 2002. 36: 47-50
9. Fedorow CA, Moon MC, Mutch WA, Grocott HP. Lumbar cerebrospinal fluid drainage for thoracoabdominal aortic surgery: Rationale and practical considerations for management. Anesth Analg. 2010. 111: 46-58
10. Jacobs MJ, Meylaerts SA, de Haan P, de Mol BA, Kalkman CJ. Assessment of spinal cord ischemia by means of evoked potential monitoring during thoracoabdominal aortic surgery. Semin Vasc Surg. 2000. 13: 299-307
11. Jacobs MJ, de Mol BA, Elenbaas T, Mess WH, Kalkman CJ, Schurink GW. Spinal cord blood supply in patients with thoracoabdominal aortic aneurysms. J Vasc Surg. 2002. 35: 30-7
12. Khan NR, Smalley Z, Nesvick CL, Lee SL, Michael LM. The use of lumbar drains in preventing spinal cord injury following thoracoabdominal aortic aneurysm repair: An updated systematic review and meta-analysis. J Neurosurg Spine. 2016. 25: 383-93
13. Martin DJ, Martin TD, Hess PJ, Daniels MJ, Feezor RJ, Lee WA. Spinal cord ischemia after TEVAR in patients with abdominal aortic aneurysms. J Vasc Surg. 2009. 49: 302-6
14. Matsuda H, Ogino H, Fukuda T, Iritani O, Sato S, Iba Y. Multidisciplinary approach to prevent spinal cord ischemia after thoracic endovascular aneurysm repair for distal descending aorta. Ann Thorac Surg. 2010. 90: 561-5
15. McHardy FE, Bayly PJ, Wyatt MG. Fatal subdural haemorrhage following lumbar spinal drainage during repair of thoraco-abdominal aneurysm. Anaesthesia. 2001. 56: 168-70
16. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: Is it preventable?. J Vasc Surg. 1999. 30: 391-7
17. Scott DA, Denton MJ. Spinal cord protection in aortic endovascular surgery. Br J Anaesth. 2016. 117: ii26-ii31
18. Sinha AC, Cheung AT. Spinal cord protection and thoracic aortic surgery. Curr Opin Anaesthesiol. 2010. 23: 95-102