- Department of Neurosurgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa, Japan
Correspondence Address:
Iku Nambu
Department of Neurosurgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa, Japan
DOI:10.4103/2152-7806.198729
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Iku Nambu, Naoyuki Uchiyama, Kouichi Misaki, Masanao Mohri, Mitsutoshi Nakada. Concurrent cervical dural and multiple perimedullary arteriovenous fistulas presenting with subarachnoid hemorrhage: The source of bleeding was invisible at initial angiography. 19-Jan-2017;8:2
How to cite this URL: Iku Nambu, Naoyuki Uchiyama, Kouichi Misaki, Masanao Mohri, Mitsutoshi Nakada. Concurrent cervical dural and multiple perimedullary arteriovenous fistulas presenting with subarachnoid hemorrhage: The source of bleeding was invisible at initial angiography. 19-Jan-2017;8:2. Available from: http://surgicalneurologyint.com/surgicalint_articles/concurrent-cervical-dural-and-multiple-perimedullary-arteriovenous-fistulas-presenting-with-subarachnoid-hemorrhage-the-source-of-bleeding-was-invisible-at-initial-angiography/
Abstract
Background:We report the concurrence of a spinal dural arteriovenous fistula (DAVF) and multiple perimedullary arteriovenous fistulas (PAVFs) presenting with subarachnoid hemorrhage (SAH). Moreover, the bleeding site was detected 1 month after onset.
Case Description:A 56-year-old man was admitted to our hospital with an SAH. A DAVF and two PAVFs were detected at the C2 level by two rounds of digital subtraction angiography. The source of bleeding, an aneurysm on the feeding artery of PAVF, was detected at the second angiogram, which was performed 1 month after the onset of SAH. The aneurysm was not demonstrated at initial angiogram because of thrombosis in the aneurysm. The DAVF was interrupted by transarterial embolization, and the two PAVFs were subsequently treated with surgery.
Conclusion:A part of the whole AVFs or the source of bleeding may be invisible in the acute stage just after hemorrhage. Repeated angiography is necessary to diagnose such complex AVFs especially in case of an SAH and treatment should be performed during the subacute stage.
Keywords: Digital subtraction angiography, spinal dural arteriovenous fistula, spinal perimedullary arteriovenous fistula, subarachnoid hemorrhage
INTRODUCTION
Spinal arteriovenous malformations are classified into four types, namely, dural arteriovenous fistulas (DAVF), epidural arteriovenous fistulas, perimedullary arteriovenous fistulas (PAVF), and intramedullary arteriovenous malformations. Here, we report a case of concurrent cervical DAVF and multiple PAVFs, presenting with subarachnoid hemorrhage (SAH), whose source of bleeding was detected 1 month after onset of the SAH. This case provides important observations regarding the diagnosis of such complex AVFs, as well as the appropriate timing of treatment for such cases with SAH.
CASE REPORT
A 56-year-old man experienced sudden onset of a severe headache, and was brought to our hospital. His Glasgow Coma Scale score was 15, and he had a stiff neck and abducens nerve paralysis.
Computed tomography of the brain revealed an SAH localized mainly at the anterior surface of the brain stem. Left vertebral artery (VA) angiography demonstrated a DAVF and two PAVFs at the C2 level [
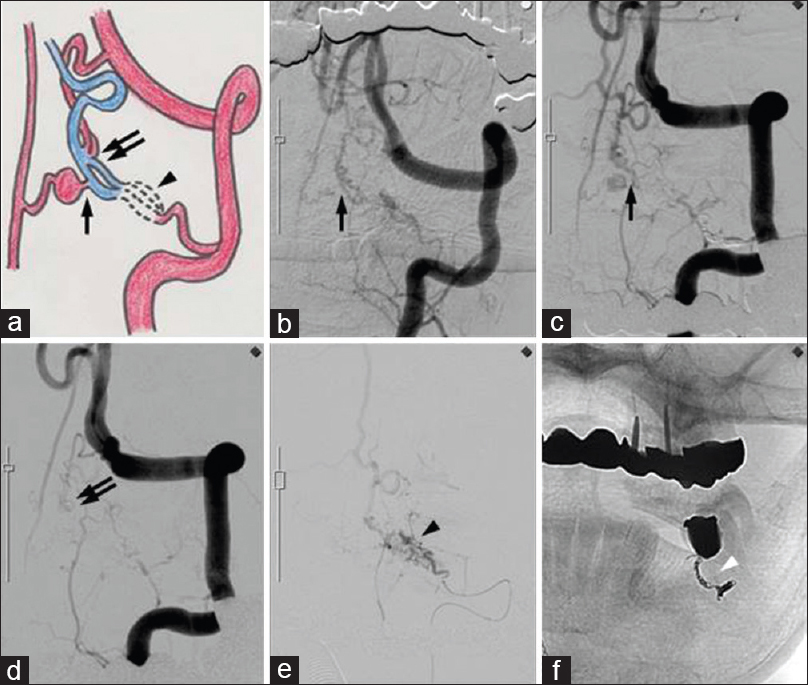
Figure 1
(a) Schematic representing a DAVF (arrowhead) and 2 PAVFs (arrow and double arrow). (b) Left vertebral angiogram showing one PAVF (arrow) that was fed by the branch of the anterior spinal artery. (c) A second angiogram that was performed 28 days after the onset of an SAH revealed an aneurysm on the distal side of the artery feeding the PAVF. (d) Another PAVF (double arrow) that was fed by the descending artery from the left vertebral artery. (e and f) Selective angiogram of the left C2 radicular artery showing a DAVF (arrowhead) and transarterial coil embolization (white arrowhead)
Surgery was performed after transarterial embolization (TAE) of the DAVF with a coil [
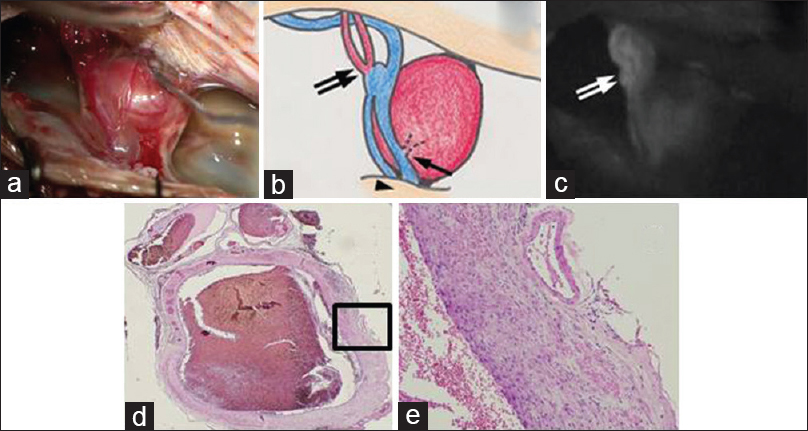
Figure 2
Intraoperative photograph (a), schematic (b), and indocyanine green videoangiography (c) demonstrating the location of 2 PAVFs (arrow and double arrow), a DAVF (arrowhead), an aneurysm on the distal side of the feeding artery, and draining veins of the DAVF and PAVFs. The histopathological features of the aneurysm (d) hematoxylin and eosin, ×20; (e) hematoxylin and eosin, ×100 demonstrating a thickened wall composed of fibrous tissue. The aneurysm retained a luminal structure
The histopathological features of the aneurysm demonstrated a thickened wall that was composed of fibrous tissue [Figure
Postoperative angiography showed complete obliteration of the DAVF and PAVFs. The patient was discharged without any neurological deficits after placement of a ventriculoperitoneal shunt for hydrocephalus.
DISCUSSION
Patients with DAVFs or PAVFs that are located in the thoracolumbar region or conus medullaris usually present with gradual worsening of symptoms, such as pain and weakness of the legs.[
The gold standard for diagnosis is DSA, which is useful for detecting feeding arteries, fistulous points, draining veins, arterial aneurysms, and varices. Moreover, important information on normal vessels that should be preserved can be obtained. Some cases of AVFs with a SAH are not identified on the first angiogram. Hayashi et al.[
Spinal AVFs are treated with surgery, endovascular embolization, or both. Surgery has low recurrence rates and endovascular embolization is the less invasive approach. In our case, it was possible to occlude the entire shunt system with endovascular embolization using N-butyl cyanoacrylate (NBCA) or Onyx from the feeder of DAVF. However, we did not choose to use these for fear of several complications. If NBCA penetrates to the distal part of the drainer and the fistulous points of PAVFs are not occluded, there is a risk that the feeder aneurysm of PAVF may rupture at high pressure. On the other hand, Onyx can occlude the proximal part of the drainer followed by two fistulas of PAVFs. However, we were concerned that Onyx with the high penetrating ability may penetrate to brainstem supplying perforators, which are invisible on angiogram. We, therefore, decided to perform a surgery in which we could detect whole vascular structures by direct viewing. In the presurgical TAE, to decrease operation blood loss, we used coils instead of glue agents to avoid occluding the intra-dural portion of vessels.
When making a diagnosis, clinicians should be aware of the possibility of the coexistence of two or more spinal cord AVFs that have similar or different characteristics in a single patient, and fistulous points should not be overlooked. Moreover, in cases with SAH, some ruptured points may not be detected at the initial angiogram for several reasons. Therefore, repeated angiography is necessary to diagnose such complex AVFs, and treatment should be performed at the subacute stage, especially in case of SAH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aviv RI, Shad A, Tomlinson G, Niemann D, Teddy PJ, Moluneux AJ. Cervical dural arteriovenous fistulae manifesting as subarachnoid hemorrhage: Report of two cases and literature review. AJNR Am J Neuroradiol. 2004. 25: 854-8
2. Cho WS, Kim KJ, Kwon OK, Kim CH, Kim J, Han MH. Clinical features and treatment outcomes of the spinal arteriovenous fistulas and malformation: Clinical article. J Neurosurg Spine. 2013. 19: 207-16
3. Dam-Hieu P, Mineo JF, Bostan A, Nonent M, Besson G. Concurrent spinal dural and intradural arteriovenous fistulas. Case report. J Neurosurg. 2001. 95: 96-9
4. Hayashi K, Takahata H, Nakamura M. Two cases of spinal arteriovenous malformation presenting with subarachnoid hemorrhage [in Japanese]. No Shinkei Geka. 2004. 32: 605-11
5. Kai Y, Hamada J, Morioka M, Yano S, Mizuno T, Kuratsu J. Arteriovenous fistulas at the cervicomedullary junction presenting with subarachnoid hemorrhage: Six case reports with special reference to the angiographic pattern of venous drainage. AJNR Am J Neuroradiol. 2005. 26: 1949-54
6. Krings T, Coenen VA, Weinzierl M, Reinges MH, Mull M, Thron A. Spinal dural arteriovenous fistula associated with a spinal perimedullary fistula: Case report. J Neurosurg Spine. 2006. 4: 241-5
7. Maslehaty H, Petridis AK, Barth H, Mehdorn HM. Diagnostic value of magnetic resonance imaging in perimesencephalin and nonperimesencephalic subarachnoid hemorrhage of unknown origin. J Neurosurg. 2011. 114: 1003-7
8. Morgalla MH, Ernemann U, Gawlowski J, Deininger M, Bitzer M, Grote EH. Recurrent spinal fistula as result of a rare combination of a perimedullary and peridural spinal fistula: Case report. Surg Neurol. 2004. 61: 347-52
9. Onda K, Yoshida Y, Watanabe K, Arai H, Okada H, Terada T. High cervical arteriovenous fistulas fed by dural and spinal arteries and draining into a single medullary vein: Report of 3 cases. J Neurosurg Spine. 2014. 20: 256-64
10. Sasaki O, Yajima N, Ichikawa A, Yamashita S, Nakamura K. Deterioration after surgical treatment of spinal dural arteriovenous fistula associated with spinal perimedullary fistula. Neurol Med Chir. 2012. 52: 516-20
11. Sato K, Endo T, Niizuma K, Fujimura M, Inoue T, Shimizu H. Concurrent dural and perimedullary arteriovenous fistulas at the craniocervical junction: Case series with special reference to angioarchitecture. J Neurosurg. 2013. 118: 451-9