- Department of Neurosurgery, Radboudumc Nijmegen, The Netherlands
- Department Oral and Maxillofacial Surgery, Radboudumc Nijmegen, The Netherlands
Correspondence Address:
H. H. K. Delye
Department of Neurosurgery, Radboudumc Nijmegen, The Netherlands
DOI:10.4103/sni.sni_17_18
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: H. H. K. Delye, W. A. Borstlap, E. J. van Lindert. Endoscopy-assisted craniosynostosis surgery followed by helmet therapy. 07-Mar-2018;9:59
How to cite this URL: H. H. K. Delye, W. A. Borstlap, E. J. van Lindert. Endoscopy-assisted craniosynostosis surgery followed by helmet therapy. 07-Mar-2018;9:59. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8806
Abstract
Background:Surgical methods to treat craniosynostosis have evolved from a simple strip craniectomy to a diverse spectrum of partial or complete cranial vault remodeling with excellent results but often with high comorbidity. Therefore, minimal invasive craniosynostosis surgery has been explored in the last few decades. The main goal of minimal invasive craniosynostosis surgery is to reduce the morbidity and invasiveness of classical surgical procedures, with equal long-term results, both functional as well as cosmetic.
Methods:To reach these goals, we adopted endoscopy-assisted craniosynostosis surgery (EACS) supplemented with helmet molding therapy in 2005.
Results:We present in detail our surgical technique used for scaphocephaly, trigonocephaly, plagiocephaly, complex multisutural, and syndromic cases of craniosynostosis.
Conclusions:We conclude that EACS with helmet therapy is a safe and suitable treatment option for any type of craniosynostosis, if performed at an early age, preferably around 3 months of age.
Keywords: Craniosynostosis, endoscopy, helmet, minimal invasive, surgical technique
INTRODUCTION
The history of the identification of different types of craniosynostosis, the underlying pathogenesis, and the subsequent development of surgical treatments for this entity reads as a very entertaining novel. In the last decade, many reports have reviewed the history, treating paradigms, and evolving surgical techniques in much detail.[
At the time Virchow stated his law, it was believed that the observed deformities in craniosynostotic skulls were a result of cessation of growth across a prematurely fused suture, with compensatory growth along nonfused sutures in a direction parallel to the affected suture, causing obstruction of normal brain growth.[
By the mid-1950s, there was a significant advance in anesthesia and blood transfusion and surgery for craniosynostosis became very safe. At that time, Moss rejected the Virchow's law and proposed his “functional matrix theory,” stating that the active growth of the underlying brain dictated the passive cranial growth along the suture lines. He proposed that the cranial base and not the suture was the primary site of abnormality, with suture fusion being a secondary consequence.[
The main goal of minimal invasive craniosynostosis surgery is to reduce the morbidity and invasiveness of classical surgical procedures, with equal long-term results, both functional and cosmetic.[
Reducing the morbidity and invasiveness can be achieved by minimizing skin incisions and tissue dissection using the smallest working space possible while keeping good visual control over the surgical field to prevent major blood loss and other complications such as dural tears.
To reach these goals, we introduced endoscopy-assisted suturectomy (ECAS) supplemented with helmet molding therapy in our centre in 2005 and gained extensive experience with this technique.[
METHODS
Surgical technique
General principles and equipment
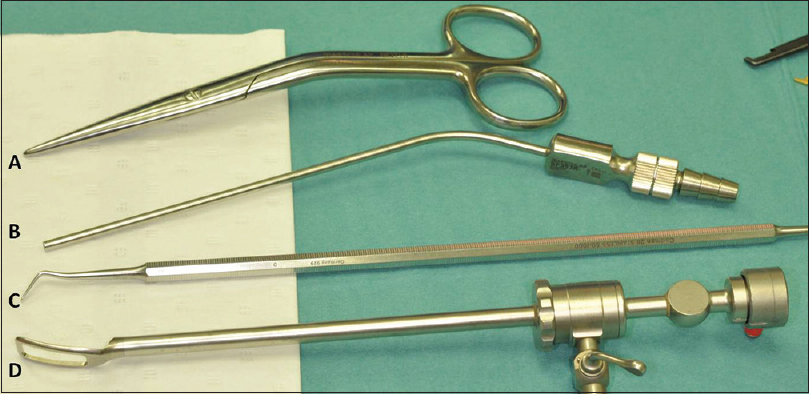
This type of surgery can be performed with a standard armamentarium including the use of an endoscope with footplate and can be considered as a simple and easy surgery when performed correctly.
To minimize blood loss, we infiltrate the skin with lidocaïne 2% or epinephrine 1:100.000. After skin incisions are made, we use monopolar cutting for galea and periosteum. The craniectomy is then initiated with a high-speed drill and continued with different rongeurs and Kerrisons. Any bleeding during surgery from the epidural space and bone edges is easily controlled with FloSeal® Matrix Hemostatic Sealant and Ostene® bone wax (Baxter Healthcare Corporation, Fremont, CA, USA).
Once a small entrance craniectomy is performed, we use a 0-degree Storz lens scope with a working shaft used for endoscopic facial lift surgery without irrigation or suction to perform dura dissection from the overlying bone and synostotic suture [
Standard anesthetic monitoring techniques including electrocardiography, noninvasive blood pressure monitoring, pulse oximetry, temperature monitoring, and blood loss monitoring are used. As blood loss and operative times are very limited (30–60 min), there is no need for a central venous line nor an arterial line. Two peripheral venous lines suffice in all cases. Antibiotic profylaxis consists of 25 mg/kg cefazolin i.v. given 20 min before skin incision. Postoperative monitoring is performed in pediatric medium care unit, with hemoglobin/hematocrit levels controlled 6 h after surgery and before dismissal the next day. Postoperative pain is treated with prophylactic paracetamol and low-dose i.v. morphine which can be tapered during the night after surgery. Because of the low level of morphinoids postoperatively and the very limited blood loss, there is no need for an urinary catheter during or after surgery. Patients can be orally fed 3–4 h after surgery. None of our patients need ICU monitoring postoperatively and almost all patients are dismissed the day after the surgery. Helmet therapy is started 2 weeks postoperatively.
Jimenez and Barone showed that the critical age for EACS seems to be 6 months.[
In syndromic cases, we aim for very early surgery at an age of 4–8 weeks, as we try to halt the progressive deformity, prevent intracranial hypertension, and simplify reconstructive surgery at a later stage. Parents are informed that cranial vault expansion and bifronto-orbital advancement procedures will still be required at a later stage. Because of the very young age and additional problems such as sleep apnea and risk of increased ICP, molding helmet therapy has not been added in these cases up to now.
Scaphocephaly
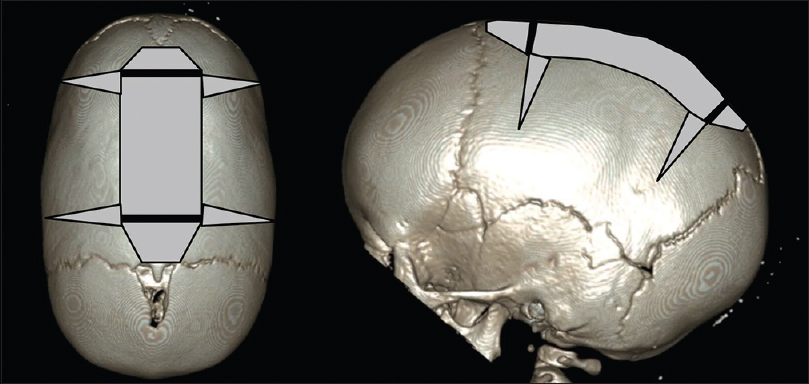
Patients are positioned in prone sphinx position, aligning the sagittal suture with the horizontal plane [
Trigonocephaly
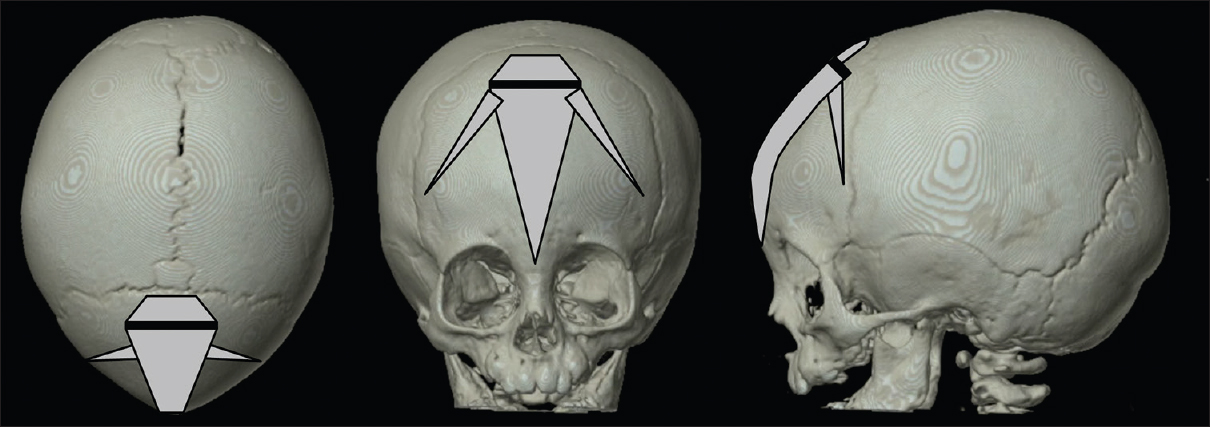
Patients are placed in a supine position, aligning the metopic suture with the horizontal plane. One skin incision of approximately 3 cm is positioned symmetrically over the metopic suture just behind the hairline [
From this skin incision, an osteoclastic craniectomy towards the anterior fontanel is performed using the high-speed drill and rongeurs after dissection and elevation of the periosteum. The length of this craniectomy can vary in case a part of the suture is still open and patent. After this, FloSeal® Matrix Hemostatic Sealant is administered for hemostasis. Then, the endoscope is introduced and dura dissection from the overlying bone is performed. The perfect visualization of the dura and operative field by the endoscope in conjunct with a parallel positioned aspirator to clear any blood allows a safe dissection of the dura without the occurrence of dural tears although the frontal bone and synostotic suture often present with deep and sharp bony ridges. Typically, some bridging veins can be found near the most anterior part of the synostotic suture and can be coagulated before rupture. When performing dura dissection, the rigid scope tends to compress the dura as dissection advances anteriorly. Of course, it is of paramount importance to avoid too much pressure on the dura. Therefore, the entrance to the subdural space with the endoscope should be as anterior as possible to overcome the curvature of the forehead. Sometimes this will demand simultaneous progressive craniectomy of the suture while performing the dura dissection. Once the dura dissection is completed, the periosteum is dissected and lifted from the suture. A triangular craniectomy is performed with a base of 3 cm, tapering down between the orbits to just above the nasion, using the endoscope to protect the dura and provide good visualization. The bone near the skull base is generally more thick and cancellous, causing more venous bleeding. This can easily be controlled by using FloSeal® Matrix Hemostatic Sealant, and Ostene® bone wax. We recently added small wedge-shaped osteotomies pointed towards the upper lateral orbital edges to our treatment protocol. We think this might assist in the outbending of the flat area just above the lateral orbital edges in trigonocephaly. Periosteum, subcutis, and cutis are closed in separate layers using resorbable sutures, Steristrips™ (3M™, Diegem, Belgium) included. A small compressive head bandage is used for 24 h. Facial and periorbital swelling is usually very mild.
Anterior plagiocephaly/brachycephaly
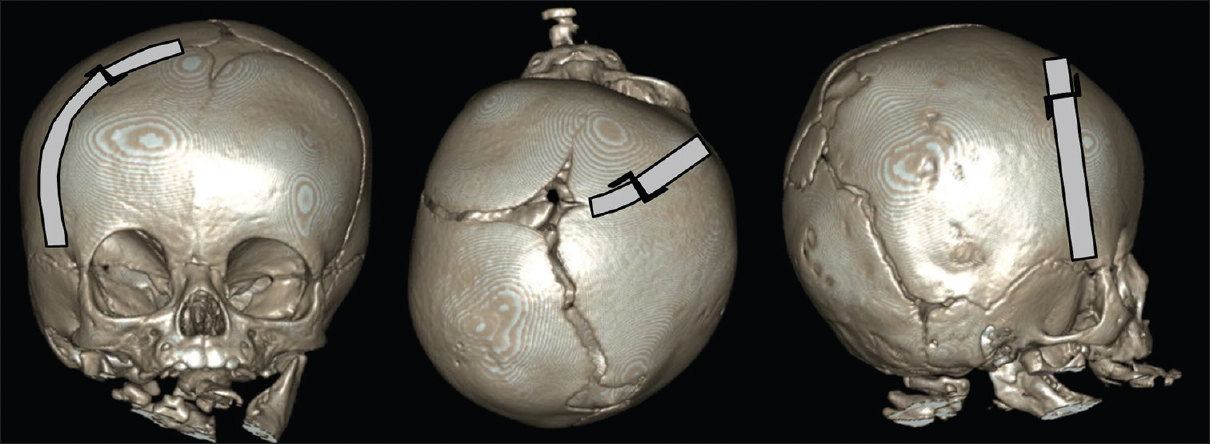
Patients are positioned in the supine position with the head contralaterally rotated in plagiocephaly cases or neutral position in brachycephaly cases. One (or two) curvilinear skin incision of approximately 3 cm wide is placed just behind the hairline over the synostotic suture(s). Depending on the hairline and the specific curvature of the forehead, we sometimes use a small zigzag incision (Harry Potter incision) to allow better skin retraction [
Multisutural and syndromic craniosynostosis
Although our experience is small for multisutural, nonsyndromic cases, we adhere to the same rationale for performing ECAS in these cases as for monosutural synostosis. Jimenez and Barone have shown that nonsyndromic multisutural craniosynostosis can be treated successfully with excellent results and reversal of the deformities.[
Recently, Jimenez and Barone reported on the endoscopic-assisted bilateral strip craniectomy of the coronal suture in an infant with Apert syndrome followed by helmet therapy.[
In our centre, we treated three Apert and two Muenke syndrome cases with EACS. However, we did so with a different goal than Jimenez et al. In syndromic craniosynostosis, we want to try to halt the progressive deformity, prevent intracranial hypertension, and simplify reconstructive surgery at a later stage by performing EACS in a very early stage (4–8 weeks of age) but without helmet molding therapy. This is a very easy and simple surgery with very low morbidity, but to our mind, it is not meant to be a replacement for conventional surgical techniques. It is rather a supplement treatment to reduce the burden of the syndrome on the infant until definitive reconstructive surgery can be performed at a later age. Some groups have started to perform posterior cranial vault expansion in a minimally invasive manner to achieve the same goal while waiting for the right time to perform definitive cranial vault reconstruction.[
Helmet therapy
To our mind, the success of EACS depends heavily on the cranial molding therapy. The helmet design, subsequent modifications, and compliance to the helmet therapy are all critical to the success of this procedure. The helmet has the ability to modify the calvarial growth pattern, and hence, the direction of growth in three dimensions. By controlling growth in most areas, the helmet focuses most of cranial growth in the areas where it is needed. By guiding the cranial growth in three dimensions, the fast developing and growing brain can act as a very effective internal distractor once suturectomy is performed. Without this guidance, e.g., due to lack of fit or noncompliance, cranial expansion occurs equally in all directions and the obtained correction after suturectomy remains incomplete.
One week after the surgery, a plaster imprint of the skull is taken, which serves as an initial template for the fabrication of the custom-made helmet and helmet therapy starts within 2 weeks after surgery. The helmet is designed to contact all areas of the cranium except where growth is desirable. Because the helmet needs to be worn 23 h daily, a perfect fit of the helmet is of paramount importance to prevent slippage or the development of pressure ulceration areas or other skin problems. Frequent follow-up by a dedicated orthotist and the craniofacial team, especially at the early stage of the therapy, ensures a perfect fit and allows for patient-specific adjustments in reaction to actual skull growth in three dimensions. This can be easily done by thermoplastic procedures until skull growth requires a new helmet. As the fastest increase in cranial volume occurs during the first two years of life, we aim for continuing helmet therapy until the age of 1–1.5 years or when normocephaly has been reached. In our series, the helmet was worn for 10 months on average.[
DISCUSSION
Recent reports focus on the embryological formation and premature closure of sutures as being the main pathogenetic cause for craniosynostosis to occur.[
By using an orthotic molding helmet, the distractive forces of the growing brain can be guided towards the preferable growing vectors in three planes. We think that EACS with helmet therapy is the next logical step in the evolution of surgical techniques for craniosynostosis as it results from the combination of new insights into the pathogenetic mechanisms at play, together with the development of new technologies.
After having performed more than 140 cases, including all types of monosutural as well as complex nonsyndromic multisutural and some syndromic cases, we consider this technique as a very safe and valuable tool in the broad range of treatment possibilities for craniosynostosis, with satisfying results [Figures
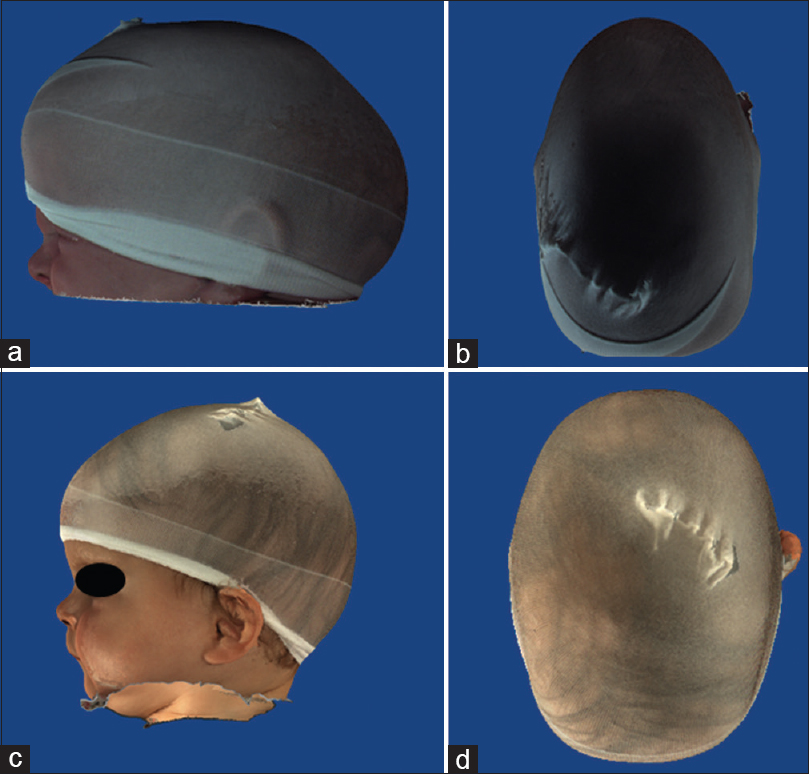
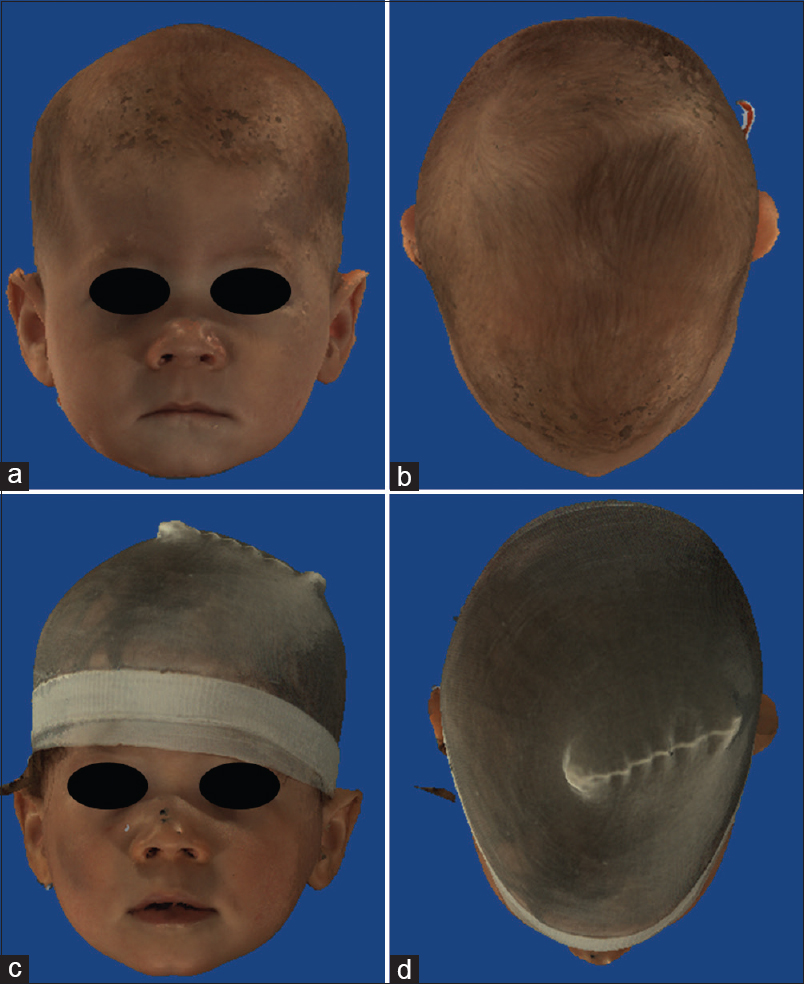
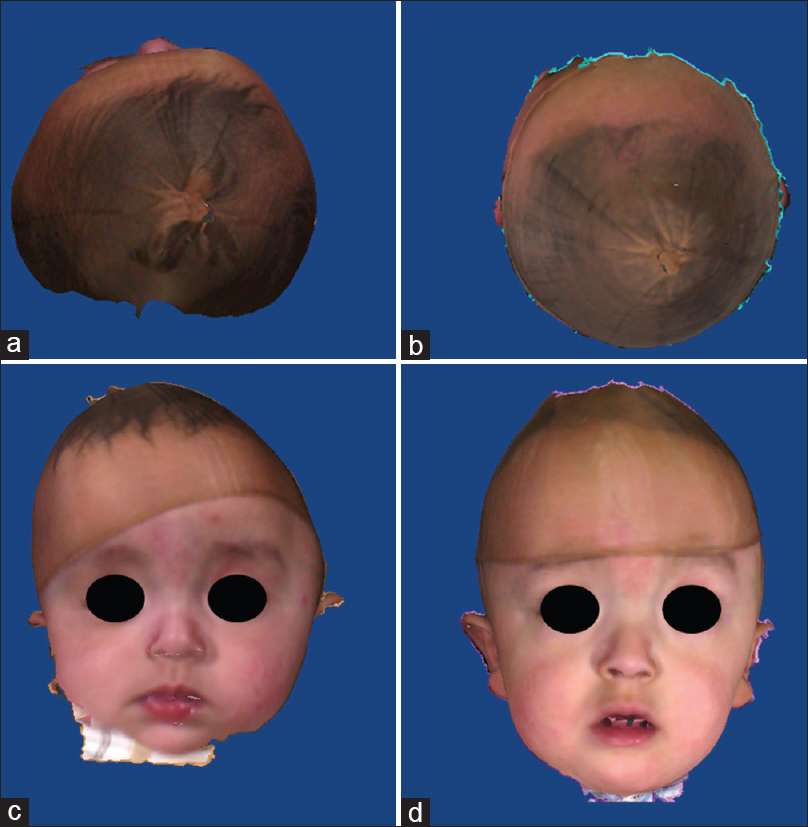
Figure 8
(a and b) pre operative 3D fotogrammetry of a patient with synostosis of left coronal and bilateral lambdoid sutures. (c and d)12 months postoperative 3D fotogrammetry of same patient. The cranial axis has almost completely aligned with the facial axis and the shape of the forehead is almost symmetrical, with perfect rounding of the occipital area
However, as experience grows and long-term follow-up data starts to be available in the literature, we think it is mandatory to evaluate the results of this technique by comparing them not only to historical data but also to other current techniques including both minimally invasive spring-assisted techniques as classical “open” techniques. Indeed, also classic “open” cranial vault reconstruction techniques have undergone some recent adjustments to reduce surgical time, blood loss, and morbidity. It is mandatory to try to evaluate whether EACS is suitable for all craniosynostosis cases, both nonsyndromic and syndromic, or only for selected subgroups.
The result of the EACS treatment depends heavily on the helmet therapy. This essential part of the treatment is often considered a major drawback of this treatment, some even call it a “complication” of this treatment (personal communication at VI World congress of Neuroendoscopy, Mumbai, 2013). In our series, helmet therapy was continued for a mean of 10 months (8–12 months). By using custom-made, very light helmets, the compliance rate of helmet therapy is very high and we never noticed pressure ulcers or major complications. In few cases, some eczema or dry skin developed, which resolved once helmet therapy was stopped. Up to now, we never had to stop helmet therapy because of intolerance. Overall, the “burden” of the helmet therapy, as reported by parents, seems to be very low. We tried to generate objective data on the burden of helmet therapy by sending an online questionnaire to all parents. The questions in the questionnaire covered all areas of the impact and were asked objectively. This does not rule out all bias, e.g., it can be that parents choosing a type of procedure are less likely to report that they made a mistake in choosing. Although only roughly one-third of all parents responded, results are mainly in favor for the helmet therapy. Almost everyone would choose again the EACS with helmet therapy and all respondents would advise others to choose this treatment.[
When looking at the key points of this treatment, we still see room for future improvements:
- Patients need to be treated as early as possible, preferably just before or at the age of 3 months to get satisfying results. Therefore, early referral to the neurosurgeon is of paramount importance. Information and education of general practitioners, pediatricians, and paramedic professionals working with children with “abnormal” head shapes (physiotherapists, manual therapists, etc.) should be actively performed to raise awareness and enlarge the therapeutic time frame through early referrals.
- Although custom available surgical instruments are sufficient to perform EACS safely and successfully, development of dedicated instruments for this type of surgery can improve efficiency and reduce surgical time and blood loss even further. Especially the development of a new type of endoscopic shaft, small dedicated instruments for hemostasis, and a new design of a small craniotome that can be easily used below the skin under endoscopic guidance would improve surgical technique.[
- Helmet design can be further refined by using 3D CAD techniques. This could allow to construct the perfect molding helmet taking into account the actual skull compared to a reference “normal” skull to define the areas and extent of desired growth and/or restriction of growth. This would make the helmet therapy more reliable and predictable, with easier, planned, adaptations. This technique is already available but at the moment is more expensive than handmade custom helmets.
Last but not least, EACS may be combined with other surgical techniques as well. Spring expansion, internal and external distraction, and orbitofrontal advancement may all be combined with EACS, wherein the combination of two techniques allows further improvement of the result.
To our mind, the biggest challenge for the coming decades in the field of craniosynostosis surgery is trying to define which surgical technique or combination of techniques – open, endoscopic, spring-assisted – yields the best results in terms of satisfying cosmetic and functional results, with the lowest morbidity, mortality and cost, for certain set parameters such as craniosynostosis subtype, degree of severity, age at presentation, gender, and genetic background.
However, the field of craniosynostosis surgery is currently limited by the lack of objective data by which to interpret and compare results.[
CONCLUSION
We think EACS with molding helmet therapy offers an excellent alternative to traditional open approaches and should be considered for children diagnosed with nonsyndromic craniosynostosis prior to 3 months of age. Based on our very limited experience, we think it might also be a meaningful add-on therapy for syndromic cases to relieve the burden of the syndrome on the infant until definitive reconstructive surgery can be performed at a later age. In our experience, the helmet molding therapy is essential for reaching good results. This therapy is well tolerated by the children and parents alike without any major complications or concerns.
Financial support and sponsorship
Nil.
Conflicts of interest
None of the authors have any conflict of interest with publication of the manuscript or an institution or product that is mentioned in the manuscript and/or is important to the outcome of the study presented.
References
1. Anderson FM, Johnson FL. Craniosynostosis; a modification in surgical treatment. Surgery. 1956. 40: 961-70
2. Arnaud E, Marchac A, Jeblaoui Y, Renier D, Di Rocco F. Spring-assisted posterior skull expansion without osteotomies. Childs Nerv Syst. 2012. 28: 1545-9
3. Berry-Candelario J, Ridgway EB, Grondin RT, Rogers GF, Proctor MR. Endoscope-assisted strip craniectomy and postoperative helmet therapy for treatment of craniosynostosis. Neurosurg Focus. 2011. 31: E5-
4. Chan JW, Stewart CL, Stalder MW, St. Hilaire H, McBride L, Moses MH. Endoscope-assisted versus open repair of craniosynostosis: A comparison of perioperative cost and risk. J Craniofac Surg. 2013. 24: 170-4
5. Delashaw JB, Persing JA, Broaddus WC, Jane JA. Cranial vault growth in craniosynostosis. J Neurosurg. 1989. 70: 159-65
6. Delye HHK, Arts S, Borstlap WA, Blok LM, Driessen JJ, Meulstee JW. Endoscopically assisted craniosynostosis surgery (EACS): The craniofacial team Nijmegen experience. J Craniomaxillofac Surg. 2016. 44: 1029-36
7. Delye H, Clijmans T, Mommaerts MY, Vandersloten J, Goffin J. Creating a normative database of age-specific 3D geometrical data, bone density and bone thickness of the developing skull: A pilot study. J Neurosurg Pediatr. 2015. 16: 687-702
8. Faber HK, Towne EB. Early craniectomy as a preventive measure in oxycephaly and allied conditions: With special reference to the prevention of blindness. Am J Med Sci. 1927. 173: 701-11
9. Faber HK, Towne EB. Early operation in premature cranial synostosis for the prevention of blindness and other sequelae. Five case reports with follow-up. J Pediatr. 1943. 22: 286-307
10. Greene CS, Winston KR. Treatment of scaphocephaly with sagittal craniectomy and biparietal morcellation. Neurosurgery. 1988. 23: 196-202
11. Hankinson TC, Fontana EJ, Anderson RCE, Feldstein NA. Surgical treatment of single-suture craniosynostosis: An argument for quantitative methods to evaluate cosmetic outcomes. J Neurosurg Pediatr. 2010. 6: 193-7
12. Ingraham FD, Alexander E, Matson DD. Clinical studies in craniosynostosis analysis of 50 cases and description of a method of surgical treatment. Surgery. 1948. 24: 518-41
13. Jacobi A. Non nocere. Med Rec. 1894. 45: 609-18
14. Jane JA, Edgerton MT, Futrell JW, Park TS. Immediate correction of sagittal synostosis. J Neurosurg. 1978. 49: 705-10
15. Jimenez DF, Barone CM. Bilateral endoscopic craniectomies in the treatment of an infant with Apert Syndrome. J Neurosurg Pediatr. 2012. 10: 310-4
16. Jimenez DF, Barone CM. Early treatment of coronal synostosis with endoscopy-assisted craniectomy and postoperative orthosis therapy: 16-year experience. J Neurosurg Pediatr. 2013. 12: 207-19
17. Jimenez DF, Barone CM. Endoscopic technique for coronal synostosis. Childs Nerv Syst. 2012. 28: 1429-32
18. Jimenez DF, Barone CM. Endoscopic technique for sagittal synostosis. Childs Nerv Syst. 2012. 28: 1333-9
19. Jimenez DF, Barone CM. Endoscopic craniectomy for early surgical correction of sagittal craniosynostosis. J Neurosurg. 1998. 88: 77-81
20. Jimenez DF, Barone CM. Endoscopy-assisted wide-vertex craniectomy, “barrel-stave” osteotomies, and postoperative helmet molding therapy in the early management of sagittal suture craniosynostosis. Neurosurg Focus. 2000. 9: e2-
21. Jimenez DF, Barone CM. Multiple-suture nonsyndromic craniosynostosis: Early and effective management using endoscopic techniques. Clinical article. J Neurosurg Pediatr. 2010. 5: 223-31
22. Jimenez DF, Barone M, Cartwright CC, Baker L. Early Management of Craniosynostosis Using Endoscopic-Assisted Strip Craniectomies and Cranial Orthotic Molding Therapy. Pediatrics. 2002. 110: 97-
23. Lane LC. Pioneer craniectomy for relief of mental imbecility due to premature sutural closure and microcephalus. JAMA. 1892. 18: 49-50
24. Lannelongue M. De la craniectomie dans la microcéphalie. Compt Rend Seances Acad Sci. 1890. 50: 1382-5
25. Lauritzen C, Sugawara Y, Kocabalkan O, Olsson R. Spring mediated dynamic craniofacial reshaping. Case report. Scand J Plast Reconstr Surg Hand Surg. 1998. 32: 331-8
26. Mehta VA, Bettegowda C, Jallo GI, Ahn ES. The evolution of surgical management for craniosynostosis. Neurosurg Focus. 2010. 29: E5-
27. Morriss-Kay GM, Wilkie AOM. Growth of the normal skull vault and its alteration in craniosynostosis: Insights from human genetics and experimental studies. J Anat. 2005. 207: 637-53
28. Moss ML. The pathogenesis of premature cranial synostosis in man. Acta Anat (Basel). 1959. 37: 351-70
29. Okada H, Gosain AK. Current approaches to management of nonsyndromic craniosynostosis. Curr Opin Otolaryngol Head Neck Surg. 2012. 20: 310-7
30. Proctor MR. Endoscopic cranial suture release for the treatment of craniosynostosis-is it the future?. J Craniofac Surg. 2012. 23: 225-8
31. Rivero-Garcia M, Marquez-Rivas J, Rueda-Torres AB, Ollero-Ortiz A. Early endoscopy-assisted treatment of multiple-suture craniosynostosis. Childs Nerv Syst. 2012. 28: 427-31
32. Sanger C, David L, Argenta L. Latest trends in minimally invasive synostosis surgery: A review. Curr Opin Otolaryngol Head Neck Surg. 2014. 22: 316-21
33. Senarath-Yapa K, Chung MT, McArdle A, Wong VW, Quarto N, Longaker MT. Craniosynostosis, Molecular pathways and future pharmacologic therapy. Organogenesis. 2012. 8: 103-13
34. Sgouros S. Skull vault growth in craniosynostosis. Childs Nerv Syst. 2005. 21: 861-70
35. Shah MN, Kane AA, Petersen JD, Woo AS, Naidoo AD, Smyth MD. Endoscopically assisted versus open repair of sagittal craniosynostosis: The St. Louis Children's Hospital experience. J Neurosurg Pediatr. 2011. 8: 165-70
36. Shillito J, Matson DD. Craniosynostosis: A review of 519 surgical patients. Pediatrics. 1968. 41: 829-53
37. Simmons DR, Peyton WT. Premature closure of the cranial sutures. J Pediatr. 1947. 31: 528-47
38. Tessier P. The definitive plastic surgical treatment of the severe facial deformities of craniofacial dysostosis. Crouzon's and Apert's diseases. Plast Reconstr Surg. 1971. 48: 419-42
39. Verhamme L. Craniosynostosis surgery: Development of a flexible, angled craniotome. MSc. Thesis, Faculty of Mechanical, Maritime and Materials Engineering, Delft University of Technology, Delft, Netherlands. 2009. p.
40. Virchow R. Uber den Cretinismus, namentlich in Franken, und uber pathologische Schädelformen. Verh Phys Med Gesell Wurzburg. 1851. 2: 230-71