- Stroke Center, Yamagata City Hospital Saiseikan, Yamagata, Japan
- Department of Emergency Medicine, Yamagata City Hospital Saiseikan, Yamagata, Japan
- Department of Neurosurgery, Yamagata University Faculty of Medicine, Yamagata, Japan.
Correspondence Address:
Atsushi Kuge, Department of Emergency Medicine, Stroke center, Yamagata City Hospital Saiseikan, Yamagata, Japan.
DOI:10.25259/SNI_163_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahiro Tanaka1, Atsushi Kuge1,2, Ryozo Saito1, Kosuke Sasaki1, Tetsu Yamaki1, Rei Kondo1, Yukihiko Sonoda3. Estimation of the rupture point of the craniovertebral junction intradural arteriovenous fistula with vessel wall magnetic resonance image and its pathological findings: A case report. 03-May-2024;15:149
How to cite this URL: Masahiro Tanaka1, Atsushi Kuge1,2, Ryozo Saito1, Kosuke Sasaki1, Tetsu Yamaki1, Rei Kondo1, Yukihiko Sonoda3. Estimation of the rupture point of the craniovertebral junction intradural arteriovenous fistula with vessel wall magnetic resonance image and its pathological findings: A case report. 03-May-2024;15:149. Available from: https://surgicalneurologyint.com/surgicalint-articles/12881/
Abstract
Background: Arteriovenous fistulas (AVFs) of the craniocervical junction (CCJ) and intradural AVFs are often associated with aneurysms and varics, and it is sometimes difficult to identify the ruptured point on radiological images. We report a case in which vessel wall magnetic resonance image (VW-MRI) was useful for identifying the ruptured point at the CCJ AVF.
Case Description: A 70-year-old man presented with a sudden onset of headache. He had Glasgow Coma Scale E4V5M6, world federation of neurosurgical societies (WFNS) Grade I. Fisher group 3 subarachnoid hemorrhage and hydrocephalus were found on head computed tomography. Cerebral angiography showed a spinal AVF at the C1 level of the cervical spine. Magnetic resonance image-enhanced motion sensitized driven equilibrium (MSDE-method showed an enhancing effect in part of the AVF draining vein, but the vascular architecture of this lesion was indeterminate. We performed continuous ventricular drainage for acute hydrocephalus and antihypertensive treatment. Cerebral angiography was performed 30days after the onset of the disease, and was revealed an aneurysmal structure in a portion of the AVF draining vein, which VW-MRI initially enhanced. On the 38th day after onset, he underwent direct surgery to occlude the AV fistula and dissect the aneurysmal structure. Histopathology showed that the aneurysmal structure was varices with lymphocytic infiltration, and hemosiderin deposition was observed near the varices.
Conclusion: Recently, VW-MRI has been reported to show an association between the enhancement of varices in dural AVF and rupture cases. VW-MRI, especially the enhanced MSDE method, may be useful in estimating the ruptured point in arteriovenous shunt disease.
Keywords: Arteriovenous fistula of craniocervical junction, Ruptured point, Subarachnoid hemorrhage, Varices, Vessel wall magnetic resonance image, CCJ dAVF, Pathological findings
INTRODUCTION
Arteriovenous fistula (AVF) of the craniocervical junction (CCJ) is a rare spinal vascular lesion classified as dural, intradural, or extradural according to vascular anatomy. Among these, intradural AVFs are reported to be more frequently associated with subarachnoid hemorrhage (SAH) (43% vs 82% vs 43%) and aneurysm-like dilation or varices in the lesion (14% vs. 74% vs. 14%).[
CASE PRESENTATION
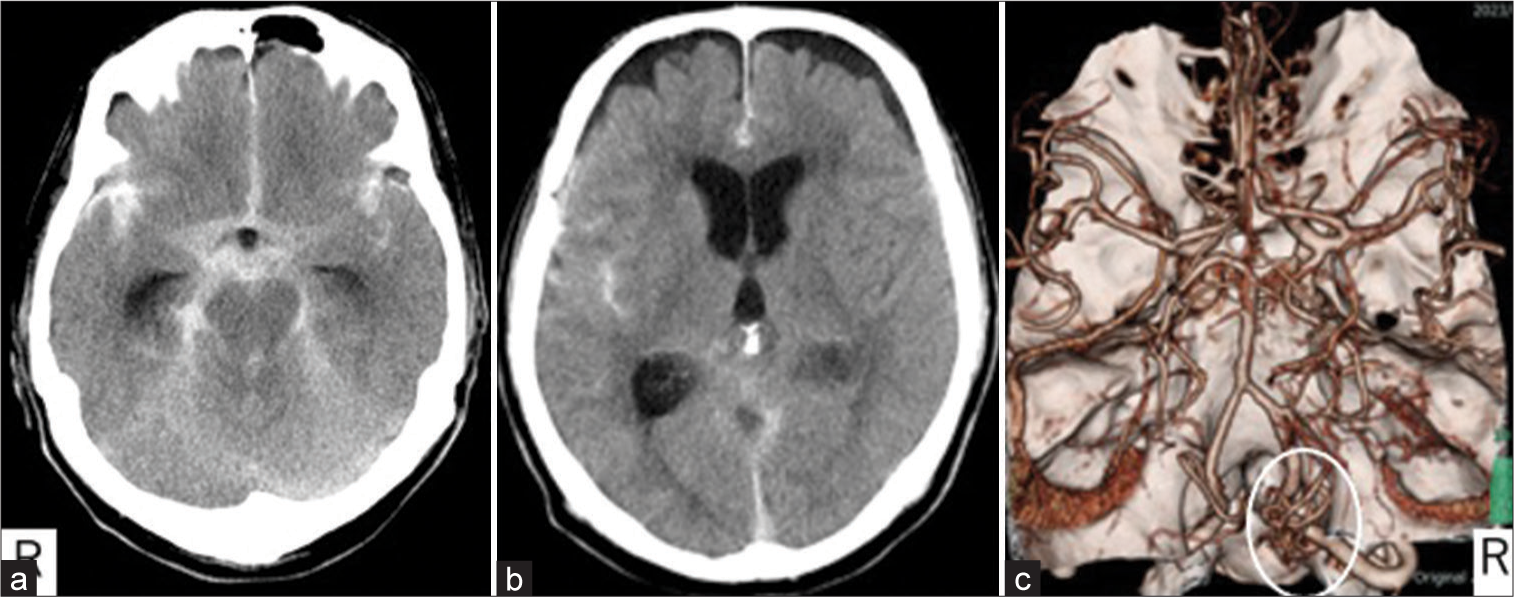
A 70-year-old male was brought to our emergency department with a complaint of sudden onset headache. He had Glasgow Coma Scale E4V5M6, WFNS grade I, at the time of arrival. A head computed tomography (CT) showed Fisher group 3 SAH and acute hydrocephalus [
Figure 2:
Initial radiological images. (a) Right vertebral angiography showing an arteriovenous fistula at the craniocervical junction. (A-P arterial phase, yellow dotted circle). (b-e) Motion-sensitized driven equilibrium shows contrast effect ventral to the spinal cord (white arrow); b and d are plain, c and e are contrast-enhanced, and d and e are magnified images of b and c (within a circle). (f) Contrast spoiled gradient recalled echo at the same level of b and c did not clearly show vascular structure (white arrow).
We diagnosed SAH due to ruptured intradural AVF at the CCJ. Feeding arteries were the C1 radicular artery, anterior spinal artery, and draining veins outflow to cranial and caudal directions. In the acute period, continuous ventricular drainage was performed. Considering the possibility that the hematoma might obscure the shunt point and that other lesions might be revealed as a source of bleeding, we decided on a wait-and-see surgical and to review radiological examinations.
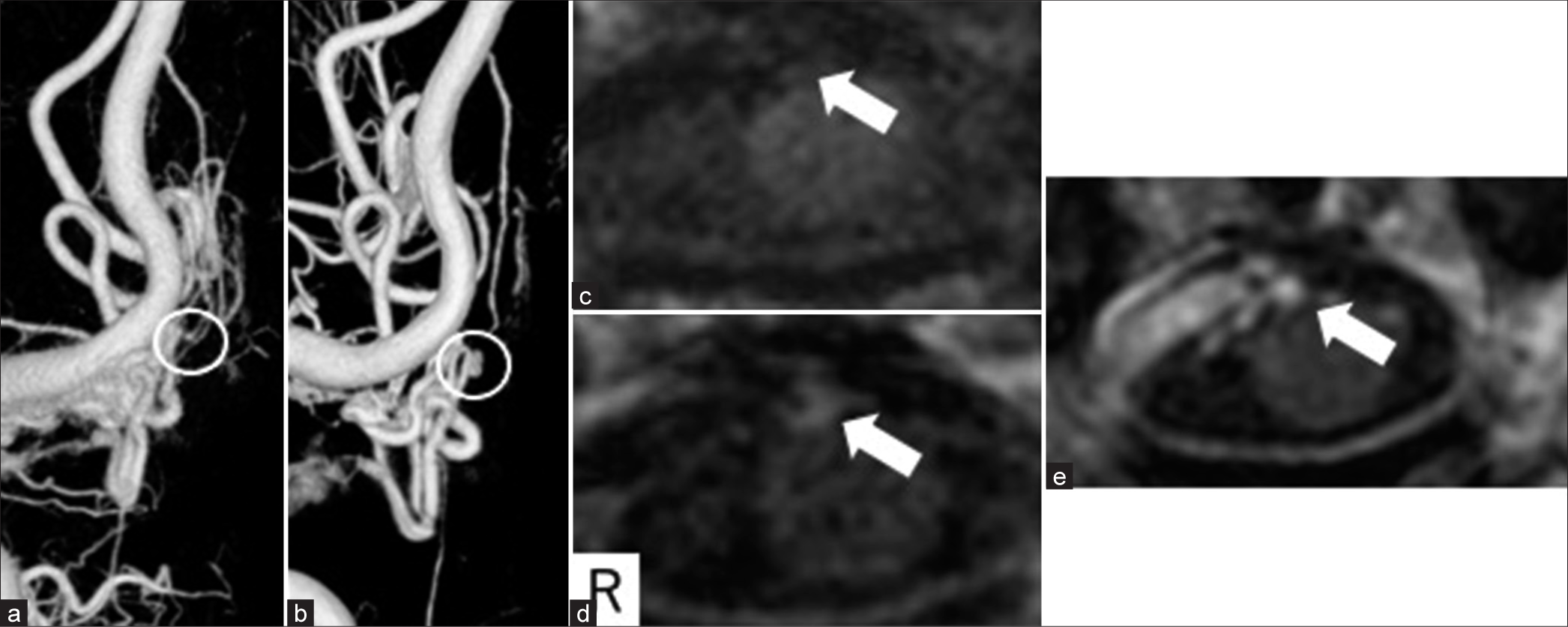
Cerebral angiography was performed again on the 26th day. A varicose structure was seen in part of the draining vein compared with initial angiography [
On the 38th day, he underwent a right suboccipital craniotomy and hemilaminectomy of the right C1. After the dural incision, a shunt point was found at the cranial side of the first cervical nerve, which was considered to be the radicular AVF [
Figure 3:
Changes in radiological images. (a) Day of onset; (b–e) 26 days after onset. (a and b) Three-dimensional digital subtraction angiography showed an aneurysmal structure in some of the draining veins (white circle). (c-e) Contrast spoiled gradient recalled echo also showed an aneurysmal structure (e), and the same level of motion-sensitized driven equilibrium showed an enhancing effect (c and d) white arrow (c-e).
Figure 4:
Intraoperative view. (a) The inside of the frame is an operation view (Three-dimensional digital subtraction angiography, white square). (b) A shunt point was noted on the cranial side of the first cervical nerve (arrow). (c and d) Double arrows indicate a draining vein. A circle indicates an aneurysmal structure.
Pathological findings
The lesion contained a muscular artery with an internal elastic lamina, a vein with a sparse muscular layer, and a vascular component with a thin wall that appeared to be a varices [
Figure 5:
Histological findings. (a) Arteriovenous fistula and aneurysmal structure (circle). (b) The aneurysmal structure was considered to be a varix because there was no internal elastic plate in the vessel wall. The vessel wall was highly thinned. (Elastica-Masson; ×40). (c) The draining vein around the varix was arterialized(arrowhead). (Elastica-Masson; ×10). (d) Lymphocytic infiltration and edematous changes in the wall of the draining vein surrounding the varix were observed (arrow). (Hematoxylin-Eosin; ×10).
Immunohistochemical staining results for
Figure 6:
Histological finding with immunohistochemical staining. (a) Draining vein around varix (Hematoxylin-Eosin; ×40). (b) Hemosiderin deposition surrounding draining veins (Berlin blue; ×40). (c) CD68-positive cells are seen in the area of hemosiderin deposition (CD68; ×40). (d) MPO staining is negative (Myeloperoxidase; ×40).
DISCUSSION
Recently, AVFs of the CCJ have been increasingly reported, but its diagnosis is difficult due to its complex vascular structure. Hiramatsu et al. reported the vascular anatomy and clinical characteristics of each and classified AVF into five categories based on the location of the feeding artery and AV shunt in the vascular anatomy.[
The presence of aneurysms and varices is associated with the development of bleeding, and some reports suggest that they are treated at the same time as the shunt point occlusion.[
After the AVF occlusion, the varices were removed after determining that the procedure could be performed safely. In our case, the lesion that was inferred to be a vascular component on initial VW-MRI could be determined to be varices on later examination, and this lesion was judged as the bleeding point intraoperatively.
It has been reported that VW-MRI cannot suppress the blood flow signal in low-velocity blood flow, such as veins and shows high signal intensity.[
However, Cord et al. reported on VW-MRI of an intracranial dural AVF (dAVF) with hemorrhagic onset with varices. In all three cases, the vein wall near the hematoma component showed a selective enhancement and was presumed to be the ruptured point.[
In our case, the reason why the draining vein of intracranial dAVF showed as an enhanced vascular component was considered to be due to arterialization of the draining vein near the shunt. Moreover, we got the pathological finding of arterialization of a continuous venous component from the varices, which supported the findings by Cord et al.[
VW-MRI is used to identify ruptured cerebral aneurysms and estimate the vulnerability of aneurysm, and inflammation of the aneurysm wall is believed to be the cause of the enhancement. Myeloperoxidase staining, which is secreted by neutrophil granulocytes, was positive in 4 out of 5 unruptured cerebral aneurysms that showed vessel wall enhancement by VW-MRI.[
Other inflammatory cells, such as macrophages, have also been implicated. Inflammation is reported to be involved in hemorrhage of cerebral AV malformations as well as cerebral aneurysms,[
In our case, a vascular lesion that had shown an enhancement on VW-MRI was found later to have a distinct vascular structure as a varices, which was presumed to be the ruptured point.
Although myeroperoxidase (MPO) staining was negative in our case, lymphocytic infiltration and CD 68 positive cells were observed near the rupture point, and the involvement of inflammation was also inferred as a factor in the positive VW-MRI finding.
Moreover, the arterialization of the venous component, which was identified in the pathology, may have contributed to the enhancement, suggesting that VW-MRI may be useful in estimating the rupture point in AV shunt disease as well.
CONCLUSION
We have experienced a case of AVF of the CCJ with SAH in which VW-MRI could identify the rupture point.
VW-MRI has the potential to identify the ruptured point not only in cerebral aneurysms but also in AV shunt disease.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bhogal P, Lansley J, Wong K, Udani SD, Uff C, Wadley J. Vessel wall enhancement of a ruptured intra-nidal aneurysm in a brain arteriovenous malformation. Interv Neuroradiol. 2019. 25: 310-4
2. Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006. 59: 72-80
3. Cord BJ, Renedo D, Santarosa C, Sujijantarat N, Antonios J, Kim JA. Vessel wall MRI in ruptured cranial Dural arteriovenous fistulas. Interv Neuroradiol. 2021. 27: 553-7
4. Endo T, Shimizu H, Sato K, Niizuma K, Kondo R, Matsumoto Y. Cervical perimedullary arteriovenous shunts: A study of 22 consecutive cases with a focus on angioarchitecture and surgical approach. Neurosurgery. 2014. 75: 238-49
5. Fujimoto S, Tanaka K, Nakatomi H, Kin T, Saito N. Three-dimensional angioarchitecture and microsurgical treatment of arteriovenous fistulas at the craniocervical junction. J Clin Neurosci. 2018. 53: 140-6
6. Hasan DM, Amans M, Tihan T, Hess C, Guo Y, Cha S. Ferumoxytol-enhanced MRI to Image inflammation within human brain arteriovenous malformations: A pilot investigation. Transl Stroke Res. 2012. 3: 166-73
7. Hiramatsu M, Sugiu K, Ishiguro T, Kiyosue H, Sato K, Takai K. Angioarchitecture of arteriovenous fistulas at the craniocervical junction: A multicenter cohort study of 54 patients. J Neurosurg. 2018. 128: 1839-49
8. Keisuke T. Update on the diagnosis and treatment of arteriovenous fistulas at the craniocervical junction: A systematic review of 92 cases. NKC. 2020. 5: 45-55
9. Larsen N, von der Brelie C, Riedel CH, Linder T, Madjidyar J, Jansen O. Vessel wall enhancement in unruptured intracranial aneurysms: An indicator for higher risk of rupture? High-resolusion MR imaging and correlated histologic findings. AJNR Am J Neuroradiol. 2018. 39: 1617-21
10. Ma Y, Song Z, Wang Y, Wang J, He C, Li G. Clinical features, treatment strategies and outcomes of craniocervical junction arteriovenous fistulas: A cohort study of 193 patients. Stroke Vasc Neurol. 2023. 9: 18-29
11. Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C. Intracranial vessel wall MRI: Principles and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol. 2017. 38: 218-29
12. Sato K, Endo T, Niizuma K, Fujimura M, Inoue T, Shimizu H. Concurrent Dural and perimedullary arteriovenous fistulas at the craniocervical junction: Case series with special reference to angioarchitecture. J Neurosurg. 2013. 118: 451-9
13. Yamaki T, Kondo R, Sato S, Mouri W, Saito G, Saito S. The usefulness of the contrast-enhanced motion-sensitized driven-equilibrium three-dimensional tubo spin echo(MSDE-3D-TSE) sequence method in cases with multiple ruptured cerebral arterial aneurysms. Surg Cereb Stroke. 2018. 46: 25-30