- Minimally-Invasive Cranial Base and Pituitary Surgery Program, Rose Ella Burkhardt Brain Tumor and Neuro-oncology Center, Cleveland Clinic Section of Skull Base Surgery, Ohio, United States.
- Section of Skull Base Surgery, Department of Neurological Surgery, Cleveland Clinic, Ohio, United States.
- Department of Anatomic Pathology, Cleveland Clinic, Ohio, United States.
- Section of Rhinology, Sinus, and Skull Base Surgery, Head and Neck Institute, Cleveland Clinic, Cleveland, Ohio, United States.
Correspondence Address:
Pablo F. Recinos
Minimally-Invasive Cranial Base and Pituitary Surgery Program, Rose Ella Burkhardt Brain Tumor and Neuro-oncology Center, Cleveland Clinic Section of Skull Base Surgery, Ohio, United States.
Section of Skull Base Surgery, Department of Neurological Surgery, Cleveland Clinic, Ohio, United States.
Section of Rhinology, Sinus, and Skull Base Surgery, Head and Neck Institute, Cleveland Clinic, Cleveland, Ohio, United States.
DOI:10.25259/SNI-30-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shahed Tish, Ghaith Habboub, Richard A. Prayson, Troy D. Woodard, Varun R. Kshettry, Pablo F. Recinos. Extraventricular neurocytoma with ganglioid differentiation of the sellar and parasellar regions in an elderly patient: A case report. 10-May-2019;10:82
How to cite this URL: Shahed Tish, Ghaith Habboub, Richard A. Prayson, Troy D. Woodard, Varun R. Kshettry, Pablo F. Recinos. Extraventricular neurocytoma with ganglioid differentiation of the sellar and parasellar regions in an elderly patient: A case report. 10-May-2019;10:82. Available from: https://surgicalneurologyint.com/surgicalint-articles/9337/
Abstract
Background: Extraventricular neurocytoma (EVN) is a rare variant of central neurocytoma which arises outside of the ventricular system. Diffuse ganglioid differentiation is a characteristic seen in a subset of these tumors which has an uncertain prognostic significance. Typically, EVN presents in children and young adults. Given the rarity of this tumor, the natural history and response to treatments remain unclear.
Case Description: We present a case of EVN with diffuse ganglioid differentiation in a 70-year-old male which arose in the midline parasellar region and extended into the third ventricle. This is the oldest such patient reported. Despite prior reports that extremes of age are associated with more aggressive behavior, the tumor in this case did not exhibit such an aggressive course.
Conclusion: In this report, we review the natural history and clinical course of this patient and summarize the literature regarding this rare pathological entity. Our patient responded well to therapy despite older age, ganglioid differentiation, and higher mitotic index.
Keywords: Extraventricular neurocytoma, ganglioid differentiation, ganglioneurocytoma, prognosis, suprasellar
INTRODUCTION
Central neurocytoma was first described by Hassoun et al. in 1982. It is typically located in the lateral ventricles near the foramen of Monro.[
EVN has the same histopathological features of central neurocytoma. These tumors are composed of uniform small cells or neurocytes with scant cytoplasm, round nuclei, and inconspicuous nucleoli.[
Herein, we present a case of a 70-year-old patient with an EVN with ganglioid differentiation located in the parasellar region and interpeduncular cistern with third ventricular extension. This would be the oldest patient reported in literature.
CASE REPORT
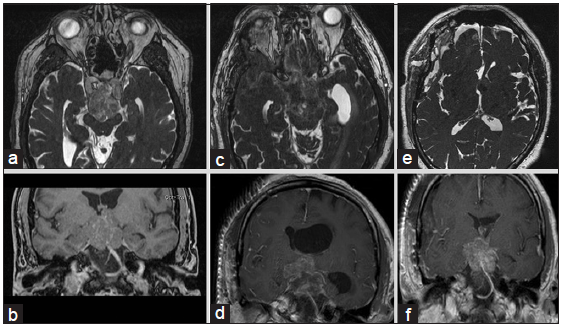
A 70-year-old man with hypertension initially presented with imbalance and dizziness and underwent magnetic resonance imaging (MRI), which demonstrated a 3.2 cm × 2.8 cm × 3.7 cm heterogeneously enhancing mass in the suprasellar region which extended to the posterior fossa resulting in mild mass effect on the midbrain and ventral pons and displacement of the optic chiasm [
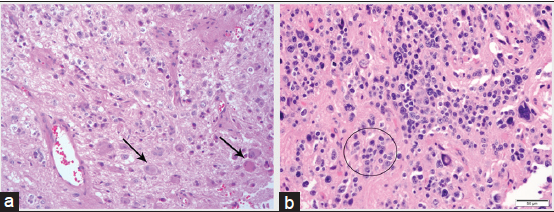
The patient was diagnosed with EVN with ganglioid differentiation. Histopathological analysis showed a moderately hypercellular tumor marked by a proliferation of small cells with scant eosinophilic cytoplasm and generally rounded nuclei. These cells were intermixed with occasional large cells with large nuclei and prominent nucleoli resembling neurons or ganglioid cells [
He was followed with serial imaging due to the favorable prognosis of the tumor. 1 year later, the patient’s tumor subsequently progressed and the patient developed short-term memory impairment as well as obstructive hydrocephalus. A right modified orbitozygomatic craniotomy and placement of a left-sided external ventricular drain was performed. Most of the tumor in the interpeduncular cistern was resected with some residual tumor in the third ventricle that did not descend. The patient had third nerve palsy postoperatively; otherwise, he was doing well. Weaning him from the external ventricular drain was difficult due to persistent obstruction at the foramen of Monro. The patient then underwent a left frontal trans-sulcal transtubular transventricular debulking of the intraventricular tumor near the foramen of Monro with septum pellucidotomy. He was successfully weaned from his ventriculostomy.
At the 6 weeks postoperative follow-up, the third nerve palsy resolved. His short-term memory and balance were significantly improved. He underwent radiation therapy with a dose of 5400 cGy in 27 fractions 5 months following surgery to treat his residual tumor. His last follow-up was at 1 year after surgery, and he continued to improve. MRI showed a slight decrease in the size of the residual tumor as compared to his preradiation MRI.
DISCUSSION
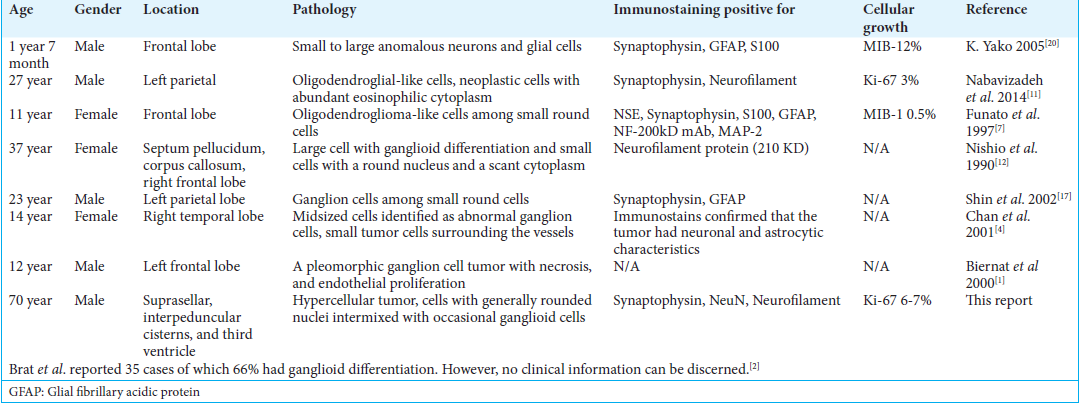
EVN with ganglioid differentiation is a rare central nervous system tumor. There are <50 documented cases and the available literature is limited to sparse case reports and case series. It has a tendency to occur in children and young adults. The mean age of presentation is 25 years with ages ranging from 2 to 70 years [
Sellar and parasellar EVN was previously reported.[
Ganglioneurocytoma is a term used to describe EVN with diffuse and convincing ganglioid differentiation.[
EVN with diffuse ganglioid differentiation may arise from any location in the brain including but not limited to frontal, temporal, and parietal lobes. All patients were children and young adults, with the exception of one case of a 37-year-old female. These tumors had low proliferative indexes and shared the same histopathological features consisting of neurocytes and ganglionic components. Almost all tumors were positive for synaptophysin. Positive staining for NSE, NeuN, neurofilament, S-100, and GFAP was described [
EVNs with ganglioid differentiation tends to exhibit a favorable prognosis but in some cases can behave more aggressively.[
CONCLUSION
EVN is a rare intracranial tumor most commonly seen in younger populations. Nevertheless, it should be considered in the differential diagnosis of an extraventricular tumor in older adults. Our patient responded well to therapy despite older age, ganglioid differentiation, and higher mitotic index, but the impact of these variables as prognostic factors remains unclear. With a growing body of literature, the natural history of EVN will be further clarified to better direct care for these patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/ their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
References
1. Biernat W, Zakrzewski K, Liberski PP. January 2000: 12 year old boy with recent onset seizures. Brain Pathol. 2000. 10: 313-4
2. Brat DJ, Scheithauer BW, Eberhart CG, Burger PC. Extraventricular neurocytomas: Pathologic features and clinical outcome. Am J Surg Pathol. 2001. 25: 1252-60
3. Buhl R, Huang H, Hugo HH, Mehdorn HM. Ganglioneurocytoma of the third ventricle. J Neurooncol. 2004. 66: 341-4
4. Chan A, McAbee G, Queenan J, Manning A. Ganglioneurocytoma mimicking a malignant tumor: Case report with a literature review of the MRI appearance of neurocytomas and gangliogliomas. J Neuroimaging. 2001. 11: 47-50
5. Choudhri O, Razavi SM, Vogel H, Li G. Atypical and rare variants of central neurocytomas. Neurosurg Clin N Am. 2015. 26: 91-8
6. Dagcinar A, Hilmi Kaya A, Ali Taşdemir H, Kuruoglu E, Sabancilar Z, Sav A. A fourth ventricular ganglioneurocytoma representing with cerebellar epilepsy: A case report and review of the literature. Eur J Paediatr Neurol. 2007. 11: 257-60
7. Funato H, Inoshita N, Okeda R, Yamamoto S, Aoyagi M. Cystic ganglioneurocytoma outside the ventricular region. Acta Neuropathol. 1997. 94: 95-8
8. Hassoun J, Gambarelli D, Grisoli F, Pellet W, Salamon G, Pellissier JF. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982. 56: 151-6
9. 10. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109 11. Nabavizadeh SA, Chawla S, Baccon J, Zhang PJ, Poptani H, Melhem ER. Extraventricular neurocytoma and ganglioneurocytoma: Advanced MR imaging, histopathological, and chromosomal findings. J Neuroimaging. 2014. 24: 613-6 12. Nishio S, Takeshita I, Fukui M. Primary cerebral ganglioneurocytoma in an adult. Cancer. 1990. 66: 358-62 13. Patil AS, Menon G, Easwer HV, Nair S. Extraventricular neurocytoma, a comprehensive review. Acta Neurochir (Wien). 2014. 156: 349-54 14. Perry A, Burton SS, Fuller GN, Robinson CA, Palmer CA, Resch L. Oligodendroglial neoplasms with ganglioglioma-like maturation: A diagnostic pitfall. Acta Neuropathol. 2010. 120: 237-52 15. Rusiecki D, Lach B, Manoranjan B, Fleming A, Ajani O, Singh SK. Progression of atypical extraventricular neurocytoma to anaplastic ganglioglioma. Hum Pathol. 2017. 59: 125-30 16. Sabatino G, Della Pepa GM, Albanese A, Lauriola L, Marchese E. Cystic ganglioneurocytoma of the lateral ventricles. Br J Neurosurg. 2014. 28: 688-90 17. Shin JH, Lee HK, Lee JK, Khang SK, Choi CG, Suh DC. MR imaging and histopathologic findings of a case of cerebral ganglioneurocytoma. Korean J Radiol. 2002. 3: 214-7 18. Wang J, Song DL, Deng L, Sun SY, Liu C, Gong DS. Extraventricular neurocytoma of the sellar region: Case report and literature review. Springerplus. 2016. 5: 987- 19. Wesseling P, van den Bent M, Perry A. Oligodendroglioma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015. 129: 809-27 20. Yako K, Nakazato Y, Hirato J, Tosaka M, Ohtani T, Ishiuchi S. Dysplastic ganglioneurocytoma with increased glucose metabolism: A heterotopia with unique histopathology. Clin Neuropathol. 2005. 24: 267-70