- Cardiovascular Prevention Center Ellasanté (Centre de Santé Ellasanté), Paris, France
- Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Correspondence Address:
Marie-Luise Mono
Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
DOI:10.4103/sni.sni_111_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Besma Sidia, Christian Saleh, Meidi El Issa, Marie-Luise Mono. Management of patent foramen ovale in patients with cryptogenic stroke: Is device closure superior to medical treatment? A brief review. 05-Jul-2018;9:132
How to cite this URL: Besma Sidia, Christian Saleh, Meidi El Issa, Marie-Luise Mono. Management of patent foramen ovale in patients with cryptogenic stroke: Is device closure superior to medical treatment? A brief review. 05-Jul-2018;9:132. Available from: http://surgicalneurologyint.com/surgicalint-articles/management-of-patent-foramen-ovale-in-patients-with-cryptogenic-stroke-is-device-closure-superior-to-medical-treatment-a-brief-review/
Abstract
Background:Recent randomized controlled trial (RCTs) comparing percutaneous closure with antithrombotic treatment in patients with patent foramen ovale (PFO) and cryptogenic stroke revealed inconsistent results. Indeed, there is still no consensus on the management of these patients, namely closure or medical therapy treatment.
Methods:To take stock of the PFO management after cryptogenic stroke, we conducted a literature review that included 16 articles dealing with different therapeutic strategies and long-term outcomes of these results.
Results:The reviewed studies showed great methodological diversity rendering an exhaustive and balanced comparison between studies difficult. Low recurrence rates under prevention regimens, crossovers, procedure- and device-related complications, as well as inappropriate patient selection might explain the inconsistency of trials. However, despite the methodological heterogeneity certain patterns could be detected. It appears that device closure as secondary prevention measure is an effective and safe procedure reducing the recurrence of neurological events in cryptogenic stroke patients
Conclusion:In cryptogenic stroke patients 60 years further trials are needed to clarify the role of PFO closure.
Keywords: Closure device, cryptogenic stroke, management, patent foramen ovale
INTRODUCTION
The prevalence of stroke remains a global scourge (approximately 12 million strokes worldwide in 2010), despite considerable therapeutic advances in recent years.[
However, not all of these PFOs are pathogenic, in 30% of these patients the PFO is an incidental finding.[
Our objective was to carry out a literature review to evaluate the role of PFO closure in different patients group.
MATERIALS AND METHODS
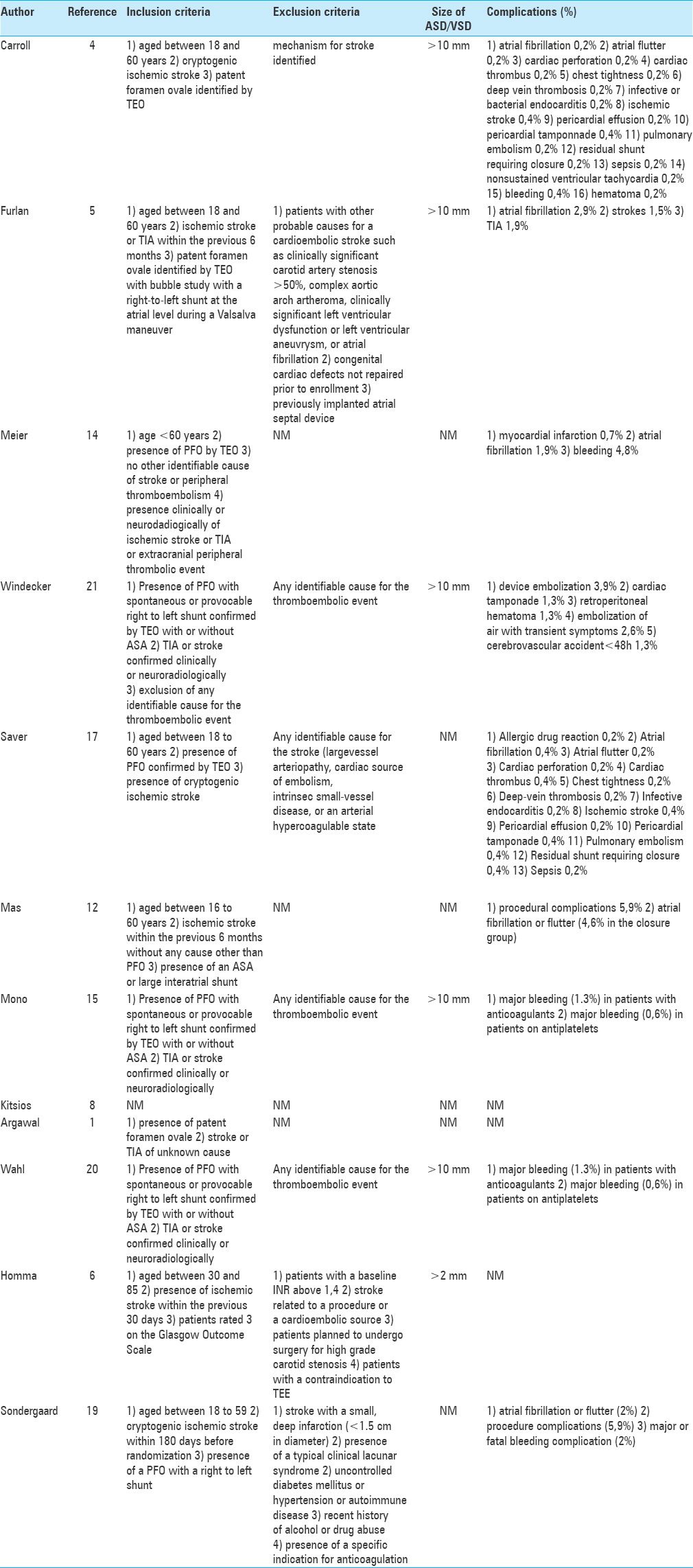
We conducted a PubMed research that included all papers concerning PFO associated with stroke. We selected only articles written in English language by using the following key words: “stroke,” “patent foramen ovale,” “atrial septal defect,” and “device closure PFO.” Key words were used in different combinations: “patent foramen ovale” and “stroke.” We chose only original studies including five randomized-controlled trials (RCT) and case series on management of PFO after cryptogenic stroke. We included 12 articles for the purpose of this review. To provide better overview we extracted the data and tabulated the data for demographics, results, and complications [Tables
RESULTS
Studies diverted greatly for size and methodology. Despite this, certain patterns could be identified through a systematic analysis of the selected studies. There were five RCTs,[
Observational studies
Kitsios et al.[
Agarwal et al.[
Wahl et al.[
Randomized studies
Furlan et al.[
Carroll et al.[
In the per-protocol cohort, primary end point in the closure group was at 0.46 events per 100 patient–years versus 1.30 events in the medical group (P = 0.03). In the as-treated cohort, the rate of primary end point was significantly different between the closure group (0.39 events per 100 patient–years) and the medical therapy group (1.45 events per 100 patients–years) (P = 0.007).
Concerning secondary end point, complete PFO closure at 6 months was reached in 72.7% of patients and 93.5% met criteria for effective closure (defined as a shunt grade of 0 or 1).
Saver et al.[
Meier et al.[
Mas et al.[
The REDUCE trial conducted by Søndergard et al.[
Safety and efficacy of PFO closure
The rate of effective closure of the PFO in the CLOSURE I trial was 86.7%. Regarding serious adverse events there were no significant differences between the two groups (16.9% in the closure group vs. 16.6% in the medical therapy group, P = 0.90). About 1.1% of the closure group patients had a thrombus in the left atrium within 6 months of whom two had a stroke. Another general complication in the closure group was atrial fibrillation. In this trial, 5.7% of closure group patients had atrial fibrillation versus 0.7% of the patients in the medical group (P < 0.001).
In the RESPECT trial, the success rate of delivery and release device was 99.1%. The rate of procedure-related or device-related serious adverse events was 4.2% in the closure group. The atrial fibrillation rate was not significantly different between the groups (3% vs. 1.5%, P = 0.13).
In the long-term follow up of RESPECT trial, the rate of pulmonary embolism in the closure group was 0.41 per 100 patient–years and 0.11 per 100 patient–years in the medical group (P = 0.04).
In the PC trial, there was no device-related thrombus in any patient. The difference observed between the two groups about atrial fibrillation was not significant, 2.9% in the closure group versus 1% in the medical group, P = 0.16.
The REDUCE trial found a significant difference concerning atrial fibrillation or flutter between the PFO closure group and the antiplatelet group (6.6% vs. 0.4%, P < 0.001, respectively).
The CLOSE trial reported 5.9% of device-related procedure in the PFO closure group. Atrial fibrillation was more common in the PFO closure group than in the antiplatelet group (4.6% vs. 0.9%, P = 0.02, respectively). The rate of serious adverse events did not differ significantly between the two groups (P = 0.56).
Wahl et al. observed in their propensity score-matched study a complete PFO closure in 82% of patients at 6 months. However, there were cases of residual shunt, small in 10% of patients, moderate in 3%, and large in 5%. A device-related thrombus was observed in two patients, resolved under anticoagulation. Major bleeding occurred in 1% of patients in the PFO closure group and in 2.9% of patients in the medically treated group (P = 0.34).
The following study did not seek to compare the different current management of PFO, but evaluated the long-term outcomes of closure.
The study from Windecker et al.[
The role of anticoagulants
It is still an open question if anticoagulants are as effective as closure? In the PICSS trial, Homma et al.[
Argawal et al.[
Data from Kitsios[
In a subgroup analysis of the RESPECT trial, there were no differences regarding risk of recurrence between the closure group and patients treated with anticoagulants (HR, 0.64; 95% CI, 0.34–1.20; P = 0.16). In the CLOSE trial, 524 were randomized into three groups: 173 patients in the closure group, 180 patients in the anticoagulation (coumadin) treatment group, and 171 patients were included in the antiplatelet group. In the intention-to-treat cohort, they observed three strokes in the anticoagulation group and seven strokes in the antiplatelet group. The authors did not proceed to a statistical significance because the study was not adequately powered to compare results in the two groups. However, other results are still expected in this study, which dates only from 2017.
Other etiologies for first-ever and recurrent stroke in patients with PFO
An intensive diagnostic work-up is needed in all patients with PFO and first-ever or recurrent stroke before evaluation a potential closure as closure can only prevent PFO-associated strokes.
In the CLOSURE I trial other etiologies could explain the recurrent cerebrovascular event (TIA or stroke) in 20 patients of the 23 in the closure group and in 22 patients of the 29 of the medical therapy group. The etiologies evoked were atrial fibrillation, a clot in the left atrium, subcortical lacunar infarction with risk factors, aortic arch atheroma, migraine complex, vasculitis, and conversion disorder. There were 12 strokes in the closure group. For three of these patients, the etiology found was atrial fibrillation and for two of them there was a thrombus device-related on the transesophageal echocardiogram (TEE). Regarding the medical therapy group, there were 13 strokes of which 1 was related to atrial fibrillation.
Mono et al.[
CONCLUSION
A PFO closure can only be effective in PFO-related strokes, which means that an extensive diagnostic work-up is needed to identify patients with “real” cryptogenic stroke who might derive benefit from PFO closure. Patients selection (e.g., inclusion of patients with lacunar stroke probably due to microangiopathy), inclusion of TIA patients as in the CLOSURE I trial, inclusion of TIA as endpoint event, low recurrence rates under prevention regimens, crossovers, procedure- and device-related complications might explain the observed inconsistency of trials. Our findings are echoed by the recent study of Ntaios et al.[
In summary, closure seems to be effective in patients >60 years with classical cryptogenic stroke, especially if there is a large shunt (Grade 2 and 3) or a coinciding ASA. However, we still do not know if closure is also effective in patients <60 years and if anticoagulants are as effective as closure or superior to antiplatelets, respectively. Atrial fibrillation is observed in up to 7% after closure and might be of clinical significance. Careful selection of clear cryptogenic stroke patients is the key to the success of the percutaneous closure procedure. In case of a recurrent event, an intensive workup is needed to rule out alternative causes of recurrence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal S, Bajaj NS, Kumbhani DJ. Meta-analysis of transcatheter closure versus medical therapy for patent foramen ovale in prevention of recurrent neurological events after presumed paradoxical embolism. JACC Cardiovasc Interv. 2012. 5: 777-89
2. Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: Incidental or pathogenic?. Stroke. 2009. 40: 2349-55
3. Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age: A study using transesophageal echocardiography. Stroke. 1993. 24: 1865-73
4. Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013. 368: 1092-100
5. Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012. 366: 991-9
6. Homma S, Di Tullio MR, Sacco RL, Mihalatos D, Li Mandri G, Mohr JP. Characteristics of patent foramen ovale associated with cryptogenic stroke: A biplane transesophageal echocardiographic study. Stroke. 1994. 25: 582-6
7. Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002. 105: 2625-31
8. Kitsios GD, Dahabreh IJ, Abu Dabrh AM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: A systematic review of observational and randomized evidence. Stroke. 2012. 43: 422-31
9. Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah , Connor M, Bennett DA. Global and regional burden of stroke during 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013. 1: e259-81
10. Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988. 318: 1148-52
11. Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001. 345: 1740-6
12. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med. 2017. 377: 1011-21
13. Masura J, Gavora P, Formanek A, Hijazi ZM. Transcatheter closure of secundum atrial septal defects using the new self-centering Amplatzer Septal Occluder. Initial Human Experience. Cath Cardiovas Diagn. 1997. 42: 388-93
14. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013. 368: 1083-91
15. Mono ML, Geister L, Galimanis A, Jung S, Praz F, Arnold M. Patent Foramen Ovale May Be Causal for the First Stroke but Unrelated to Subsequent Ischemic Events. Stroke. 2011. 42: 2891-5
16. Ntaios G, Papavasileiou V, Sagris D, Makaritsis K, Vemmos K, Steiner T. Closure of Patent Foramen Ovale Versus Medical Therapy in Patients With Cryptogenic Stroke or Transient Ischemic Attack: Updated Systematic Review and Meta-Analysis. Stroke. 2018. 49: 412-8
17. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med. 2017. 377: 1022-32
18. Serena J, Segura T, Perez-Ayuso MJ, Bassaganyas J, Molins A, Dávalos A. The need to quantify right-to-left shunt in acute ischemic stroke: A case-control study. Stroke. 1998. 29: 1322-8
19. Søndergaard L, Kasner SE, Rhodes JF. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med. 2017. 377: 1033-42
20. Wahl A, Jüni P, Mono ML. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation. 2012. 125: 803-12
21. Windecker S, Wahl A, Chatterjee T, Garachemani A, Eberli FR, Seiler C. Percutaneous Closure of Patent Foramen Ovale in Patients With Paradoxical Embolism. Circulation. 2000. 101: 893-8