- Department of Neurosurgery, Lifeline Hospital and Research Center, Azamgarh, Uttar Pradesh, India
- Department of Pathology, Dr Lal path Labs, National Reference Laboratory, New Delhi, Delhi, India
- Department of Neuroanesthesia and Neuro Critical Care, Lifeline Hospital and Research Center, Azamgarh, Uttar Pradesh, India.
Correspondence Address:
Anoop Kumar Singh, Department of Neurosurgery, Lifeline Hospital and Research Center, Azamgarh, Uttar Pradesh, India.
DOI:10.25259/SNI_150_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anoop Kumar Singh1, Shilpi2, Gayatri Kumari3, Rajiv Tangri2. Medically responsive amyloidogenic giant sellar-parasellar prolactinoma. 12-Apr-2024;15:129
How to cite this URL: Anoop Kumar Singh1, Shilpi2, Gayatri Kumari3, Rajiv Tangri2. Medically responsive amyloidogenic giant sellar-parasellar prolactinoma. 12-Apr-2024;15:129. Available from: https://surgicalneurologyint.com/surgicalint-articles/12853/
Abstract
Background: Giant prolactinomas are rare; among them, the amyloidogenic variant, prolactinomas with extensive spherical amyloid deposits, are rare, with only 30 cases reported with recommendations of surgical management contrary to the routine prolactinoma’s medical management.
Case Description: We report here a case of giant amyloidogenic prolactinoma in a 32-year-old male patient who had a very atypical presentation in terms of clinical, radiological, and pathological features and responded to dopamine agonist therapy like a normal prolactinoma.
Conclusion: Amyloidogenic giant prolactinomas are rare. Contrary to usual belief, even they remain medically responsive; however, more literature is required to decide their ideal management.
Keywords: Amyloidogenic, Macroadenoma, Parasellar, Pituitary, Prolactinoma
INTRODUCTION
Giant pituitary spherical amyloidogenic macroadenomas are rare tumors, with only approximately 30 cases reported so far.[
CASE REPORT
A 32-year-old male patient presented with an 8-month history of generalized tonic-clonic seizures. The patient was conscious, oriented, and without any neurological deficit.
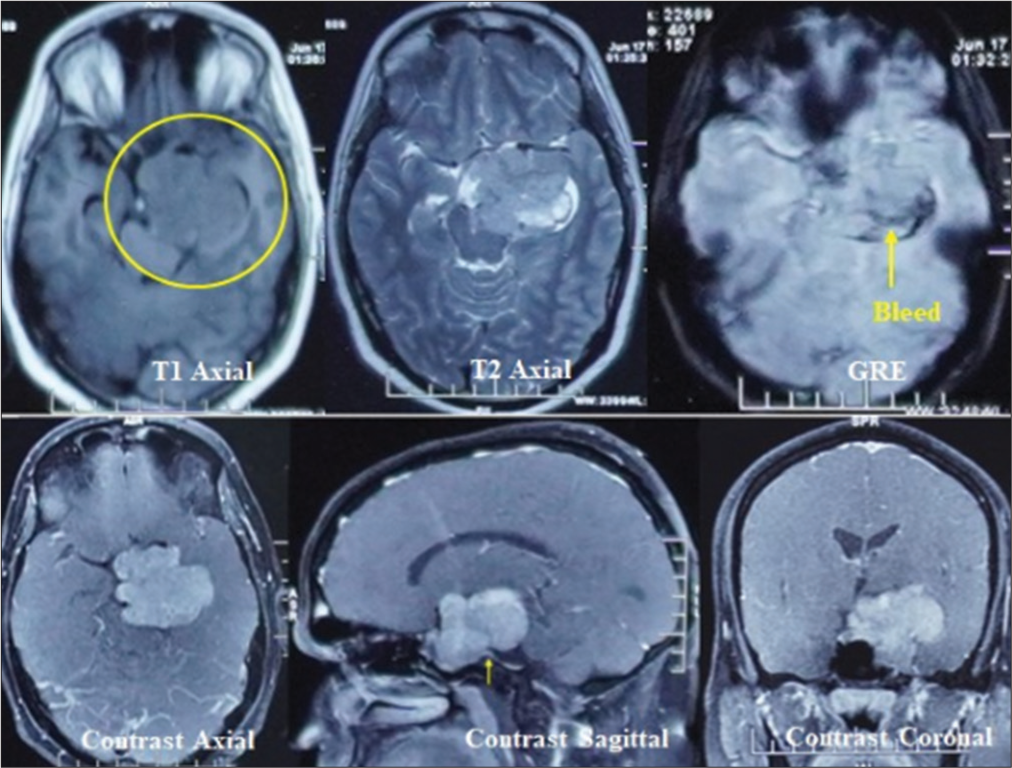
A contrast magnetic resonance imaging (MRI) brain revealed a significant extra-axial lobulated altered signal intensity left parasellar mass showing T1 isointense with few hyperintense foci, T2 and fluid attenuated inversion recovery heterogeneous signal intensity with areas of cystic degeneration and necrosis, small areas of bleed on gradient-echo, and post-contrast heterogeneous enhancement but no intra-axial edema. A positive dural-tail sign was also evident at the posterior basal dura.
Medially, the lesion was extending into the sella, and the pituitary gland was not visualized separately. The lesion extended posteriorly in the prepontine cistern and left ambient cistern, compressing the brainstem and encasing the vertebral artery. Laterally, it involved the left cavernous sinus, displacing the left cavernous internal carotid artery (ICA) and producing a mass effect on the left medial temporal lobe. Anteriorly, the lesion extends to the left orbital apex, abutting the left optic nerve and compressing the optic chiasm [
A two-stage surgery, a transcranial followed by transsphenoidal surgery, was planned, with the fronto-temporoorbito-zygomatic (FTOZ) approach as the first stage, being a significant left parasellar component. With the patient’s consent, a left FTOZ craniotomy was performed. A soft, suckable, moderately vascular lesion was found during surgery, encasing the left third cranial nerve, left internal carotid, and the basilar artery and filling the left cavernous sinus. A maximum safe resection was performed to avoid a neuro-vascular deficit.
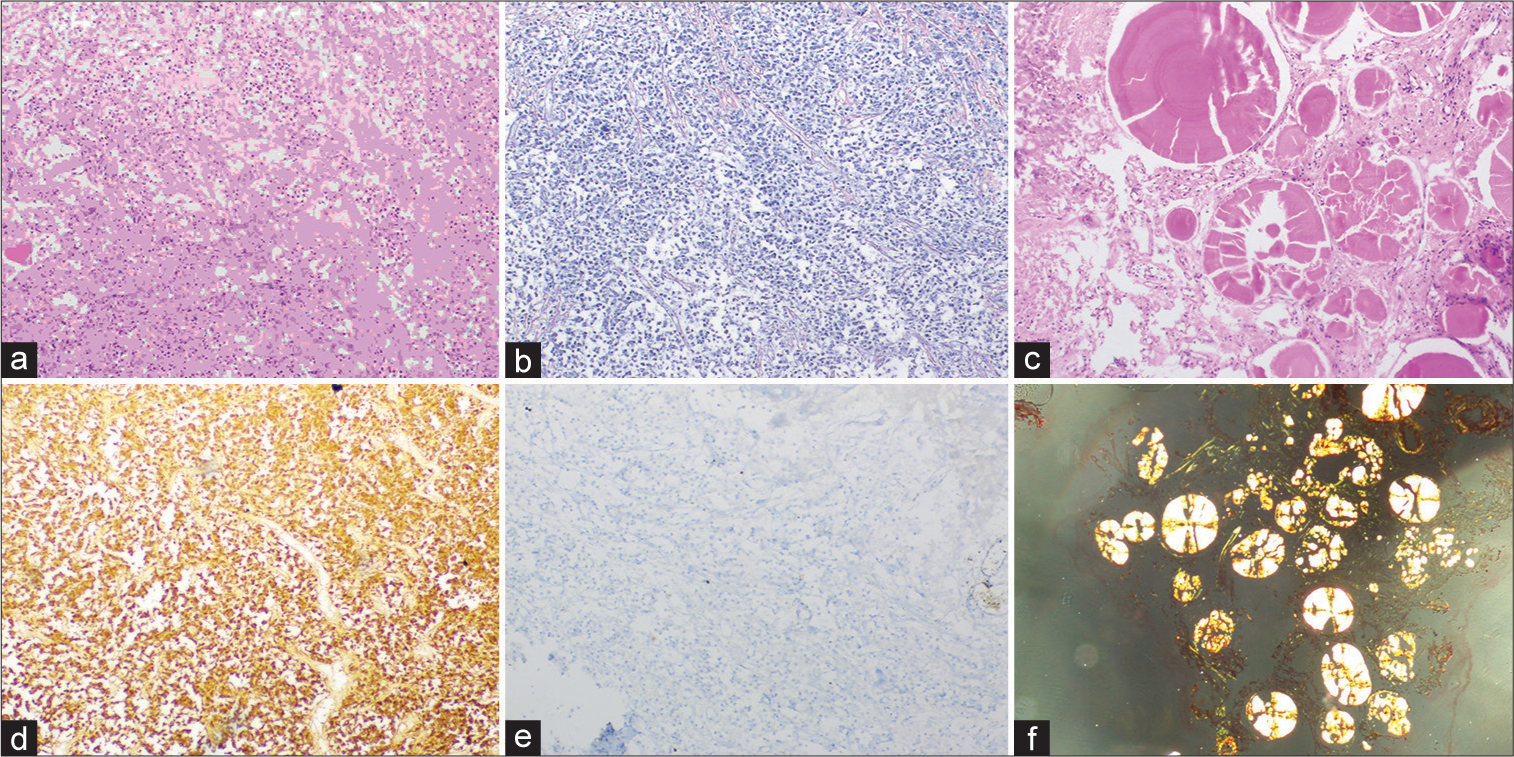
On histopathology, formalin-fixed paraffin-embedded sections showed sheets and nests of round to polygonal cells with moderate to abundant eosinophilic granular cytoplasm and central nuclei. Interrupted reticulin network identified on reticulin stain. Multiple foci showed extracellular deposits of eosinophilic laminated spheroids with radial striations, which showed apple green birefringence with Congo red stain suggestive of amyloid. Strong prolactin (PRL) immunoreactivity with a low Ki67 labeling index, whereas no reaction with other pituitary hormones and cytokeratin renders a diagnosis of prolactinoma [
Figure 2:
(a) Histomorphology showing sheets of round/polygonal cells with eosinophilic granular cytoplasm and central nuclei. (b) Interrupted reticulin network- reticulin stain. (c) Spherical amyloid globules-H&E stain. (d) Immunohistochemistry-prolactin stain. (e) Low Ki67 labeling index. (f) Congo red stain highlighting apple-green birefringence.
Retrospectively, in view of pathological findings, a hormone analysis revealed an elevated PRL level of more than 2000 ng/mL. Rest pituitary hormone levels were within normal limits, confirming the lesion to be a giant prolactinoma.
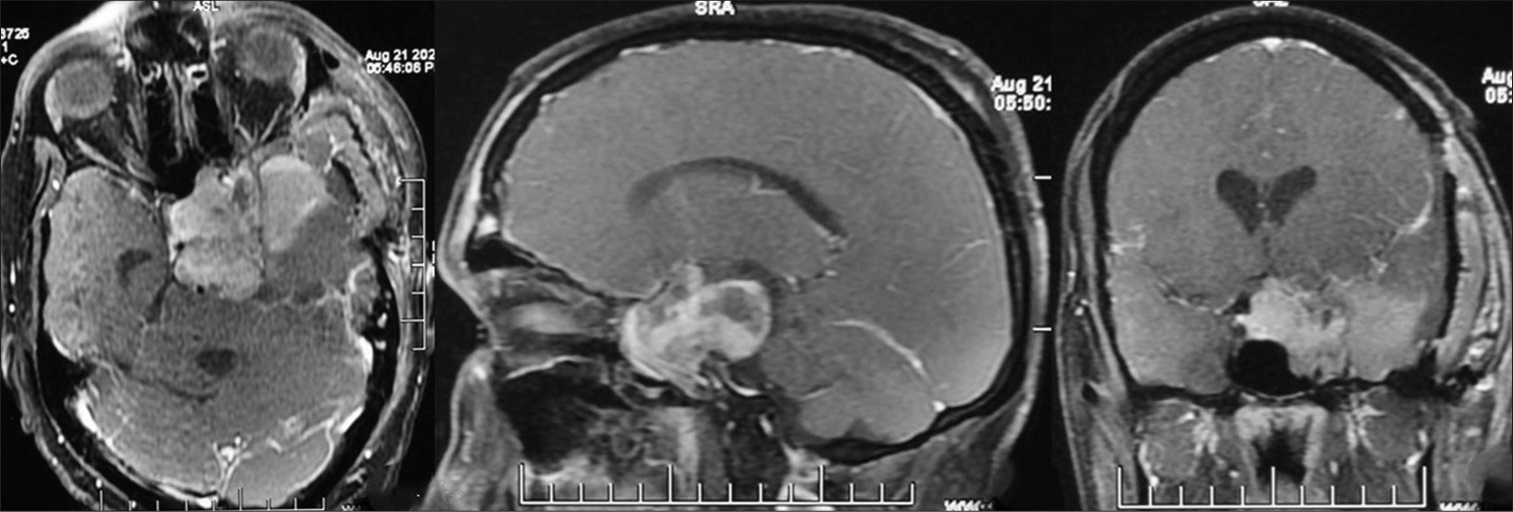
Postoperative MRI showed good tumor decompression but a significant residual in the sellar-suprasellar region [
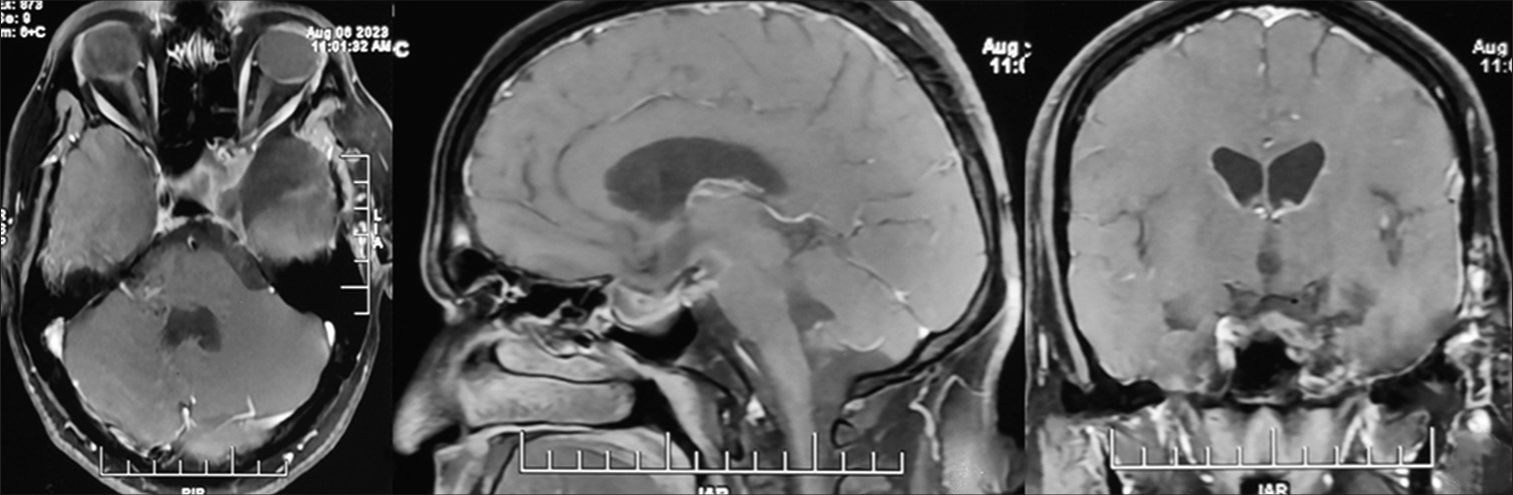
The patient remained asymptomatic; serial imaging showed a gradual decline in adenoma size [
DISCUSSION
Pituitary macroadenomas originate within the anterior pituitary and are among the more common abnormalities of the sellar region, accounting for 10–15% of intracranial tumors.[
In most reported prolactinoma cases, the male patients presented with a history of visual disturbances, headache, and erectile dysfunction, whereas the females presented with irregular menses, headache, and galactorrhea.[
It’s not unusual in parasellar lesions to find a surprising diagnosis after surgery. As in our case, the lesion imaging features, being epicenter in a parasellar location, contrast enhancement, and positive dural tail sign in the background of seizure as a presenting feature and the absence of visual complaints, we considered a chief possibility of meningioma.[
The tumor histopathology revealed the presence of spherical amyloid deposits, and immunohistochemical staining was strongly positive for PRL, showing amyloid deposit secretion directly by PRL-secreting cells. Amyloid deposits are commonly observed in pituitary adenomas in two histological patterns-stellate and spherical deposition. Stellate amyloid deposits are a perivascular fibrillar pattern of deposits found in all endocrine types of pituitary adenoma and are more common. Spherical deposits are rarely seen, almost exclusively in prolactinomas and rarely in growth hormone, adrenocorticotropic hormone-secreting, and nonsecretory adenomas.[
These deposits are difficult to diagnose radiologically preoperatively and often remain undiagnosed. However, Martin et al. described adenomas, which usually have isointense signals relative to gray matter on both T1 and T2 sequences, showing signal intensity loss on T2-weighted images as an amyloid characteristic feature on MRI.[
According to the Pituitary Society International Consensus Statement (2023), medical therapy with dopamine agonists is a first-line treatment for giant prolactinomas with recommendations of debulking surgery in resistant cases to improve the medical treatment responsiveness.[
Given prevailing literature, our observation of a medically responsive residual lesion pointed out that these giant tumors may have a characteristic of dopamine agonist resistance, maybe because of size or some inherent nature, and become medically responsive after surgical debulking like other giant prolactinomas; hence, further study and more literature will be required before labeling these amyloidogenic lesions of the pituitary as purely surgical ones.
CONCLUSION
Prolactinomas with spherical amyloid deposits are very rare. The unique pathological characteristic of these pituitary adenomas, especially in the background of atypical presentations, makes further analysis and more literature essential in deciding patient management in this particular subgroup.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bononi PL, Martinez AJ, Nelson PB, Amico JA. Amyloid deposits in a prolactin-producing pituitary adenoma. J Endocrinol Invest. 1993. 16: 339-43
2. Gul S, Bahadir B, Dusak A, Kalayci M, Edebali N, Acikgoz B. Spherical amyloid deposition in a prolactin-producing pituitary adenoma. Neuropathology. 2009. 29: 81-4
3. Gatto F, Perez-Rivas LG, Olarescu NC, Khandeva P, Chachlaki K, Trivellin G. Diagnosis and treatment of parasellar lesions. Neuroendocrinology. 2020. 110: 728-39
4. Gupta M, Grover N, Sen R. Spherical amyloid deposition in prolactinoma. Indian J Pathol Microbiol. 2020. 63: 491-2
5. Iglesias P, Arcano K, Berrocal VR, Bernal C, Villabona C, Díez JJ. Giant prolactinoma in men: Clinical features and therapeutic outcomes. Horm Metab Res. 2018. 50: 791-6
6. Li D, Wang Y, Tan H, Luo P, Yu Y. A giant invasive macroprolactinoma with recurrent nasal bleeding as the first clinical presentation: Case report and review of literature. BMC Endocr Disord. 2023. 23: 107
7. Levine SN, Ishaq S, Nanda A, Wilson JD, Gonzalez-Toledo E. Occurrence of extensive spherical amyloid deposits in a prolactin-secreting pituitary macroadenoma: A radiologicpathologic correlation. Ann Diagn Pathol. 2013. 17: 361-6
8. Martin SW, Lefton DR, Pinto RS, Rosenblum M, Elowitz E. MR imaging characteristics of amyloid deposits in pituitary adenoma. AJNR Am J Neuroradiol. 2002. 23: 368-70
9. Petersenn S, Fleseriu M, Casanueva FF, Giustina A, Biermasz N, Biller BM. Diagnosis and management of prolactin-secreting pituitary adenomas: A Pituitary Society international Consensus Statement. Nat Rev Endocrinol. 2023. 19: 722-40
10. Prabhu S, Prabhu S. Pituitary prolactinoma with amyloid deposits: Surgery or dopamine agonists? review of previous reports and new recommendations for management. Asian J Neurosurg. 2019. 14: 754-8