- Department of Neuroscience, Winthrop Neuroscience, Winthrop University Hospital, Mineola, New York, USA
Correspondence Address:
Nancy E. Epstein
Department of Neuroscience, Winthrop Neuroscience, Winthrop University Hospital, Mineola, New York, USA
DOI:10.4103/sni.sni_38_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. Neurological complications of lumbar and cervical dural punctures with a focus on epidural injections. 26-Apr-2017;8:60
How to cite this URL: Nancy E. Epstein. Neurological complications of lumbar and cervical dural punctures with a focus on epidural injections. 26-Apr-2017;8:60. Available from: http://surgicalneurologyint.com/surgicalint-articles/neurological-complications-of-lumbar-and-cervical-dural-punctures-with-a-focus-on-epidural-injections/
Abstract
Background:Various types of lumbar dural punctures may contribute to neurological injury. The etiologies of dural injury include; inadvertent dural punctures due to epidurals placed for labor anesthesia, epidural steroid injections (ESI/transforaminal TESI; approximately 9 million ESI performed in the US per year), deliberate placement of intradural pain devices, and spontaneous cerebrospinal fluid (CSF) fistulas. Resulting neurological complications may include; spinal headaches/intracranial hypotension, subdural hematomas, and 6th nerve cranial palsies. Furthermore, uniquely in the cervical spine, inadvertent cervical dural punctures attributed to cervcial ESI (CESI) may lead to intramedullary spinal cord injuries (e.g. resulting in monoparesis to quadriplegia) or spinal cord strokes due to intravascular/vertebral artery injections.
Methods/Results:In 8 studies, inadvertent lumbar dural punctures contributed to intracranial hypotension, subdural hematomas, and double vision/6th cranial nerve palsies. In 5 of the 6 studies, inadvertent dural punctures occurring during CESI were responsible for intramedullary spinal cord injuries, or direct intravascular/vertebral injections resulting in monoplegia/quadriplegia.
Conclusions:Inadvertent lumbar dural punctures led to multiple neurological complications including intracranial hypotension, subdural hematomas, and double vision/6th cranial nerve palsies. Uniquely, inadvertent cervical dural punctures solely due to CESI directly resulted in intramedullary spinal cord injuries or cord stroked and monoplegia/quadriplegia attributed to intravascular/vertebral artery injections. The potential neurological risks/complications/adverse events attributed to lumbar and cervical ESI must be taken into account before spine surgeons and others order these procedures.
Keywords: Cervical, complications, epidural steroid injection, lumbar, monoplegia, new deficit, quadriplegia, risks, sixth cranial nerve palsy
INTRODUCTION
Various types of lumbar and cervical dural punctures result in significant neurological injury. In the lumbar spine, lumbar dural punctures may be attributed to; inadvertent epidural injections for labor/anesthesia, deliberate epidural steroid injections (ESI) [(lumbar LESI)/transforaminal TFESI)], direct placement of intradural/intrathecal pain devices, deliberately when placing intradural pain devices, or in rare instances, spontaneously. Neurological complications attributed to these lumbar punctures include; spinal headaches/intracranial hypotension, subdural hematomas, and 6th cranial nerve palsies/double vision. Cervical dural punctures solely attributed to cervical ESI (CESI), uniquely risked monoparesis/quadrliplegia due to intramedullary spinal cord injections/injury, or stroke due to intravascular vertebral artery injections. The neurological complications/adverse events (AE)/risks of lumbar and cervical ESI must be carefully considered when spine surgeons and other specialists order these procedures often for minimal spinal complaints.
LUMBAR SPINAL EPIDURAL INJECTIONS IN US
Look at how often non FDA (Food and Drug Administration) approved ESI are being perfomed in the U.S. Manchikanti et al. estimated that approximately 9 million epidural steroid injections are performed in the US per year.[
INADVERTENT LUMBAR INTRADURAL INJECTIONS OCCURRING DURING ATTEMPTED EPIDURAL INJECTIONS FOR ANESTHESIA DURING LABOR AND DELIVERY
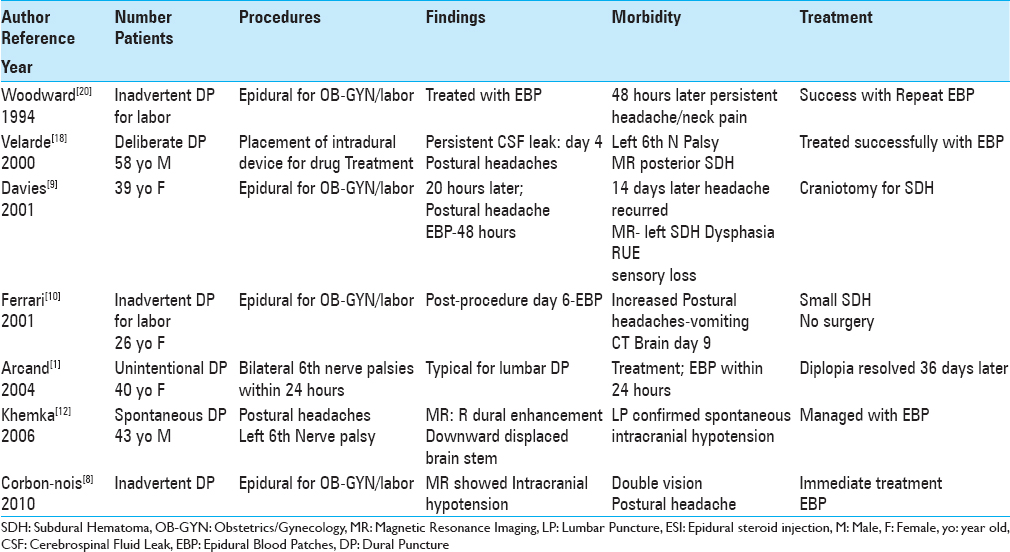
Inadvertent lumbar dural punctures resulted in intracranial hypotension, subdural hematomas, and/or 6th cranial nerve palsies in 4 patients undergoing attempted epidural spinal injections performed to deliver anesthesia during labor/delivery [
THREE CASES OF DOUBLE VISION/6TH CRANIAL NERVE PALSIES DUE TO DELIBERATE, INADVERTENT, OR SPONTANEOUS LUMBAR DURAL PUNCTURES
Three cases of double vision/6th cranial nerve palsies were variously attributed to deliberate, inadvertent, or spontaneous lumbar dural punctures [
CERVICAL EPIDURAL SPINAL INJECTIONS
Bureau et al. noted the “catastrophic complications” that may be seen with cervical ESI (BUREAU 2014).[
FOUR CERVICAL EPIDURAL STEROID INJECTIONS RESPONSIBLE FOR INTRAMEDULLARY SPINAL CORD INJURIES
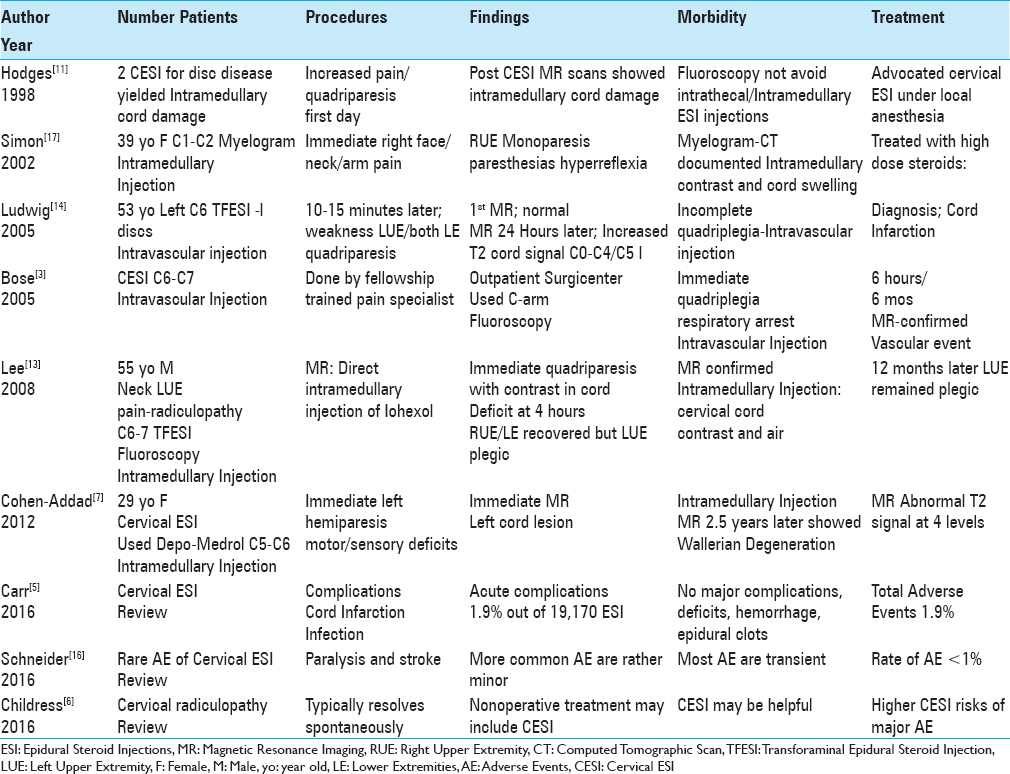
Four patients in 3 studies developed monoplegia/quadriplegia following intramedullary spinal cord injections occurring during cervical ESI (CESI) [
TWO CASES OF CERVICAL EPIDURAL STEROID INJECTIONS RESULTING IN INTRAVASCULAR VERTEBRAL ARTERY INJECTIONS AND SPINAL CORD STROKES
In two cases, CESI resulted in inadvertent intravascular vertebral artery injections, responsible for to irreversible quadriparesis/quadriplegia [
ONE CASE OF CERVICAL MYELOGRAPHY LEADING TO AN INTRAMEDULLARY SPINAL CORD INJECTION/QUADRIPLEGIA
A 39-year-old female underwent a C1-C2 myelogram and immediately noted “right-side face, neck, and arm pain and paresthesias” accompanied by right arm weakness/hyperreflexia [
THREE STUDIES MINIMIZE OR LARGELY DENY COMPLICATIONS OF CERVICAL EPIDURAL STEROID INJECTIONS
Three additional studies minimized or largely denied that significant complications resulted from cervical ESI [
CONCLUSION
Various types of lumbar and cervical dural punctures result in significant neurological injury. In the lumbar spine, these may occur during attempted epidural anesthesia for labor/delivery, while performing LESI/TLESI, during placement intradural pain devices, or spontaneously. They can result in intracranial hypotension, subdural hematomas, and double vision/6th cranial nerve palsies. In the cervical spine, all dural punctures were attributed to CESI resulting in monoplegia/quadriplegia directly attributed to intramedullary spinal cord injuries or vertebral artery injections/cord strokes. The neurological complications attributed to lumbar and more notably cervical ESI must be carefully considered when choosing to perfom any type of epidural injection, particularly for those with too often, minimal complaints.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arcand G, Girard F, McCormack M, Chouinard P, Boudreault D, Williams S. Bilateral sixth cranial nerve palsy after unintentional dural puncture. Can J Anaesth. 2004. 51: 821-3
2. Bhatia A, Flamer D, Shah PS, Cohen SP. Transforaminal Epidural Steroid Injections for Treating Lumbosacral Radicular Pain from Herniated Intervertebral Discs: A Systematic Review and Meta-Analysis. Anesth Analg. 2016. 122: 857-70
3. Bose B. Quadriparesis following cervical epidural steroid injections: Case report and review of the literature. Spine J. 2005. 5: 558-63
4. Bureau NJ, Moser T, Dagher JH, Shedid D, Li M, Brassard P. Transforaminal versus intra-articular facet corticosteroid injections for the treatment of cervical radiculopathy: A randomized, double-blind, controlled study. AJNR Am J Neuroradiol. 2014. 35: 1467-74
5. Carr CM, Plastaras CT, Pingree MJ, Smuck M, Maus TP, Geske JR. Immediate Adverse Events in Interventional Pain Procedures: A Multi-Institutional Study. Pain Med. 2016. p.
6. Childress MA, Becker BA. Nonoperative Management of Cervical Radiculopathy. Am Fam Physician. 2016. 93: 746-54
7. Cohen-Adad J, Buchbinder B, Oaklander AL. Cervical spinal cord injection of epidural corticosteroids: Comprehensive longitudinal study including multiparametric magnetic resonance imaging. Pain. 2012. 153: 2292-9
8. Corbonnois G, O’Neill T, Brabis-Henner A, Schmitt E, Hubert I, Bouaziz H. Unrecognized dural puncture during epidural analgesia in obstetrics later confirmed by brain imaging. Ann Fr Anesth Reanim. 2010. 29: 584-8
9. Davies JM, Murphy A, Smith M, O’Sullivan G. Subdural haematoma after dural puncture headache treated by epidural blood patch. Br J Anaesth. 2001. 86: 720-3
10. Ferrari L, De Sevin F, Vigué JP, Granry JC, Preckel MP. Intracranial subdural hematoma after obstetric dural puncture. Ann Fr Anesth Reanim. 2001. 20: 563-6
11. Hodges SD, Castleberg RL, Miller T, Ward R, Thornburg C. Cervical epidural steroid injection with intrinsic spinal cord damage. Two case reports. Spine. 1998. 23: 2137-42
12. Khemka S, Mearza AA. Isolated sixth nerve palsy secondary to spontaneous intracranial hypotension. Eur J Neurol. 2006. 13: 1264-5
13. Lee JH, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Spinal cord injury produced by direct damage during cervical transforaminal epidural injection. Reg Anesth Pain Med. 2008. 33: 377-9
14. Ludwig MA, Burns SP. Spinal cord infarction following cervical transforaminal epidural injection: A case report. Spine. 2005. 30: E266-8
15. Manchikanti L, Pampati V, Hirsch JA. Retrospective cohort study of usage patterns of epidural injections for spinal pain in the US fee-for-service Medicare population from 2000 to 2014. BMJ. 2016. 6: e013042-
16. Schneider B, Zheng P, Mattie R, Kennedy DJ. Safety of epidural steroid injections. Expert Opin Drug Saf. 2016. 15: 1031-9
17. Simon SL, Abrahams JM, Sean Grady M, LeRoux PD, Rushton SA. Intramedullary injection of contrast into the cervical spinal cord during cervical myelography: A case report. Spine. 2002. 27: E274-7
18. Velarde CA, Zuniga RE, Leon RF, Abram SE. Cranial nerve palsy and intracranial subdural hematoma following implantation of intrathecal drug delivery device. Reg Anesth Pain Med. 2000. 25: 76-8
19. Wald JT, Maus TP, Geske JR, Carter RE, Diehn FE, Kaufmann TJ. Safety and efficacy of CT-guided transforaminal cervical epidural steroid injections using a posterior approach. AJNR Am J Neuroradiol. 2012. 33: 415-9
20. Woodward WM, Levy DM, Dixon AM. Exacerbation of post-dural puncture headache after epidural blood patch. Can J Anaesth. 1994. 41: 628-31
21. Yang S, Werner BC, Cancienne JM, Hassanzadeh H, Shimer AL, Shen FH. Preoperative epidural injections are associated with increased risk of infection after single-level lumbar decompression. Spine J. 2016. 16: 191-6