- Department of Neurosurgery, Acibadem City Clinic University Hospital Tokuda, Sofia, Bulgaria.
Correspondence Address:
Donika Ivova Vezirska, Department of Neurosurgery, Acibadem City Clinic University Hospital Tokuda, Sofia, Bulgaria.
DOI:10.25259/SNI_42_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Asen Hristov Cekov, Donika Ivova Vezirska, Christo Tzekov Tzekov, Vladimir Stefanov Nakov. Parasagittal meningeal hemangiopericytoma/solitary fibrous tumor: Two case reports and a literature review. 19-Apr-2024;15:133
How to cite this URL: Asen Hristov Cekov, Donika Ivova Vezirska, Christo Tzekov Tzekov, Vladimir Stefanov Nakov. Parasagittal meningeal hemangiopericytoma/solitary fibrous tumor: Two case reports and a literature review. 19-Apr-2024;15:133. Available from: https://surgicalneurologyint.com/surgicalint-articles/12866/
Abstract
Background: Solitary fibrous tumor/meningeal hemangiopericytoma (SFT/M-HPC) is a rare neoplasm which accounts for around 1% of the intracranial masses. This pathology has a high risk for recurrence and metastasis to distant locations such as the liver, lungs, and bones. Precise diagnosis necessitates detailed histopathological examination.
Case Description: We present two case reports of SFT/M-HPC. The first case is a 44-year-old female who presented with headache, nausea, vomiting, and frontal ataxia for several months. Imaging findings showed a large parasagittal extra-axial mass with compression of the frontal horns of both lateral ventricles. She underwent gross total resection with an uncomplicated postoperative period. The patient had no recurrent tumors or distal metastases in the follow-up period of 5 years. The second case is a 48-year-old male who presented with right-sided hemianopsia and hemiparesis. Computed tomography (CT) scans revealed a large parieto-occipital extra-axial mass with superior sagittal sinus engulfment and dislocation of the interhemispheric fissure. He underwent gross total resection with an uncomplicated postoperative period. Six years later, he presented with right-sided weakness. CT scan showed a multifocal recurrent mass at the previous location. He underwent subtotal resection with an uncomplicated postoperative period.
Conclusion: SFT/M-HPC should be considered when presented with a meningioma-like tumor mass on preoperative imaging. Immunohistochemical study is crucial for the correct diagnosis. Strict long-term follow-up examinations and regular magnetic resonance imaging scans are key to preventing the appearance of metastases and large recurrent masses.
Keywords: Meningeal hemangiopericytoma, Meningioma, Parasagittal, Solitary fibrous tumor
INTRODUCTION
Solitary fibrous tumor/meningeal hemangiopericytoma (SFT/M-HPC) is a rare tumor which accounts for around 1% of the intracranial masses[
The SFT/M-HPC closely mimics meningioma both clinically and radiologically. However, this pathology is much more aggressive and tends to high recurrence rate and metastasis to distant locations both intra- and extracranially.[
Here, we report two cases of parasagittal SFT/M-HPC and shortly review diagnosis tips, imaging and pathology findings, and current trends in patient management.
CASE PRESENTATION
Case 1
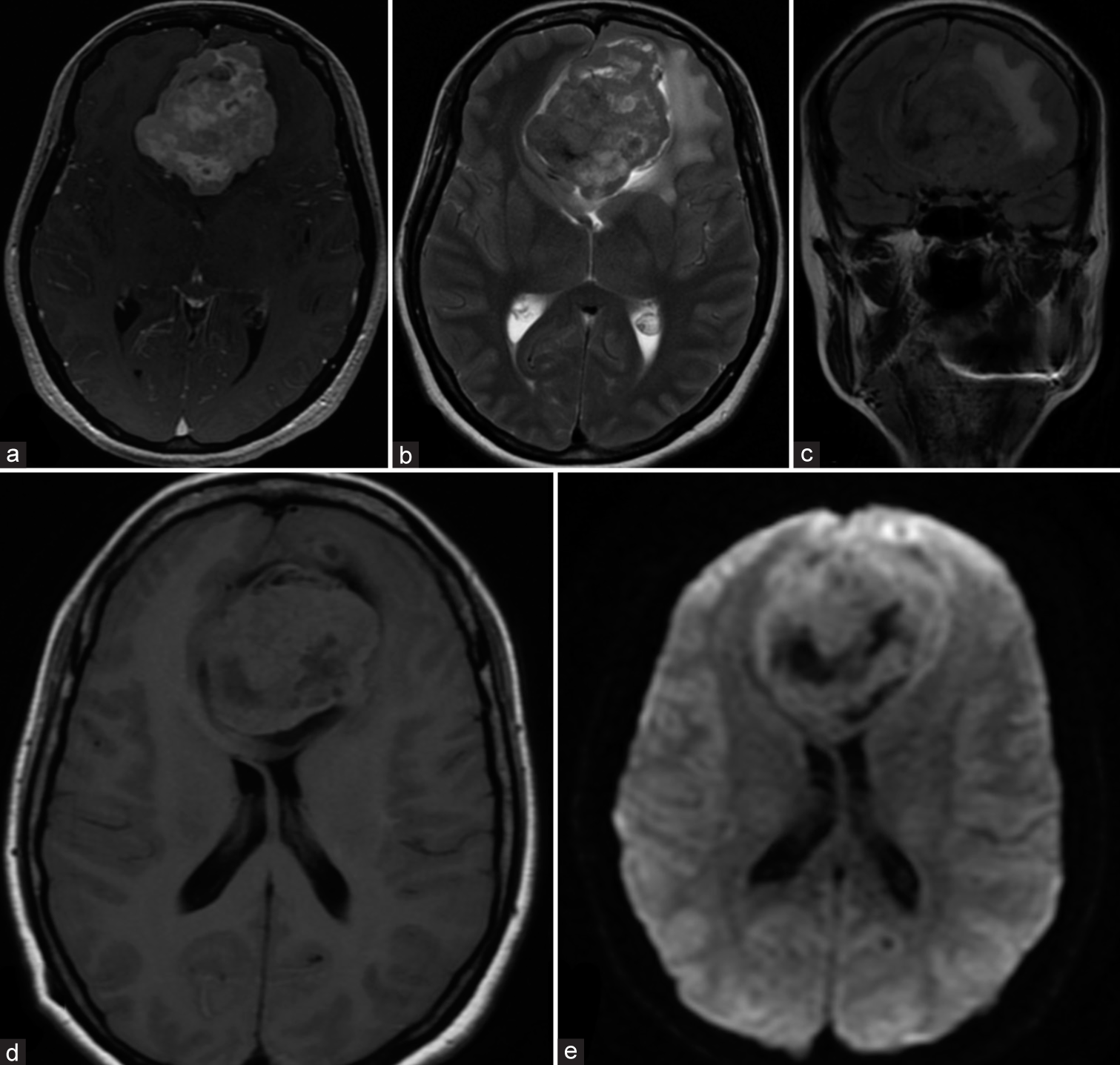
The first case is a 44-year-old female who presented with headache, nausea, vomiting, and frontal ataxia. Magnetic resonance imaging (MRI) scans showed a large extraaxial (65 × 60 mm) parasagittal mass with compression of the frontal lobe and the frontal horns of both lateral ventricles [
Figure 1:
Appearance of the lesion on (a) Axial three-dimensional fast spoiled gradient-echo sequence with contrast matter; (b) Axial T2 PROPELLER sequence; (c) Coronal T2 FLAIR PROPELLER sequence; (d) Axial T1 FLAIR sequence; (e) Axial diffusion-weighted imaging sequence with apparent diffusion coefficient map. FLAIR: Fluid-attenuated inversion recovery.
She underwent bifrontal craniotomy with ligation of the anterior third of the superior sagittal sinus. The depth and borders of the lesion were explored through ultrasound (US). The excised tumor was partially aspirable and formed a well-demarcated arachnoid plane – the feeding vessels were identified to be direct branches of the ACA. Profuse intraoperative bleeding occurred, and blood loss was estimated at around 500 mL – however, this was managed with bipolar coagulation and hemostatic agents. The dura over the lesion was prophylactically excised, and duraplasty was achieved with 7.5 × 7.5 mm Lyoplant® Onlay (Aesculap, BBraun, Melsungen, Germany). Standard layered closure was performed.
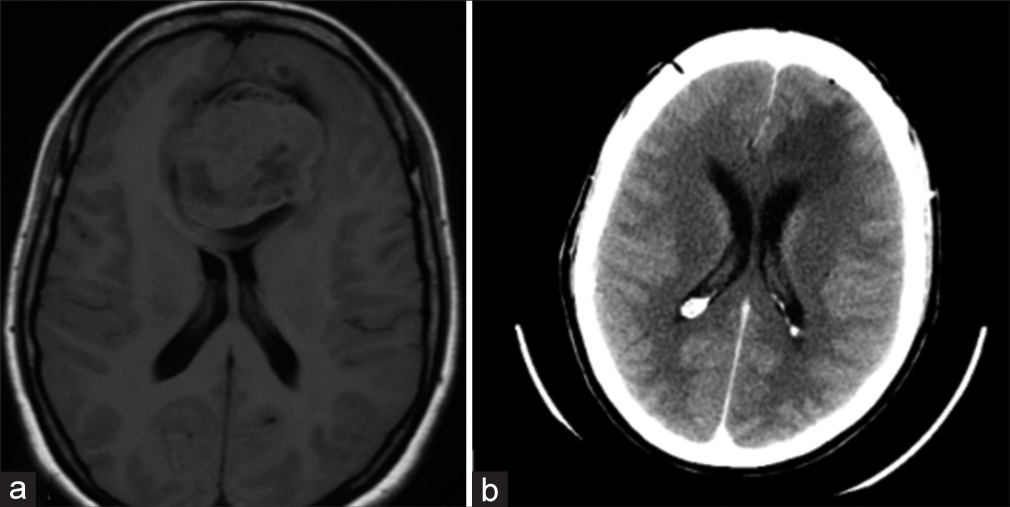
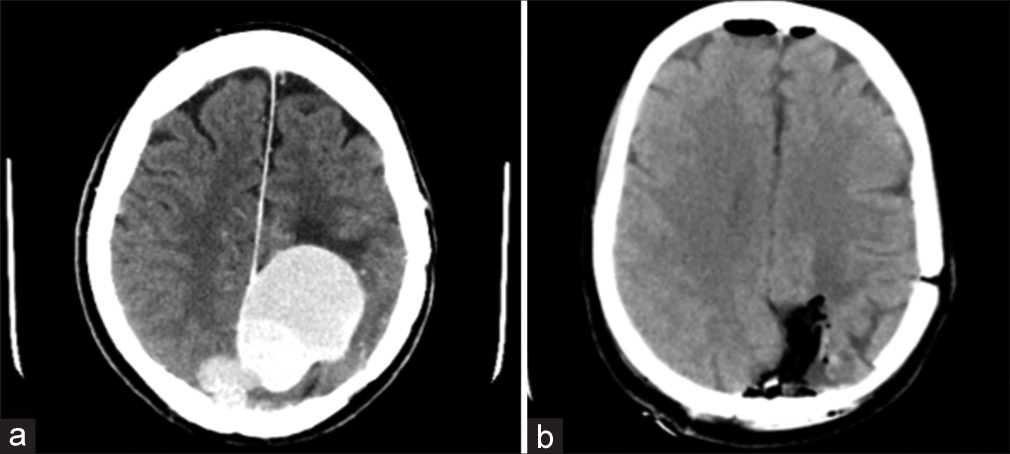
On postoperative day 1, a head computed tomography (CT) scan was performed – it revealed subtotal resection of the lesion [
Histopathological examination revealed a malignant tumor with diffuse structure and high cellularity, which consisted of cells with poor cytoplasm and large ovoid vesicular nuclei. Small parts of the probe showed pseudorosettes. Additional immunohistochemical processing showed more than five mitoses/ten high-power fields, as well as EMA-, NSE-, CD34-, and vimentin-positive staining and strongly positive staining for Bcl2 and CD99. Finally, the lesion was diagnosed as the WHO grade III meningeal HPC.
A multidisciplinary committee agreed on postoperative radiotherapy. Adjuvant field radiotherapy with 60 Gy in 2 Gy/fraction was delivered through three-dimensional conformal radiation therapy. Only mild side effects, such as erythema and alopecia, were observed.
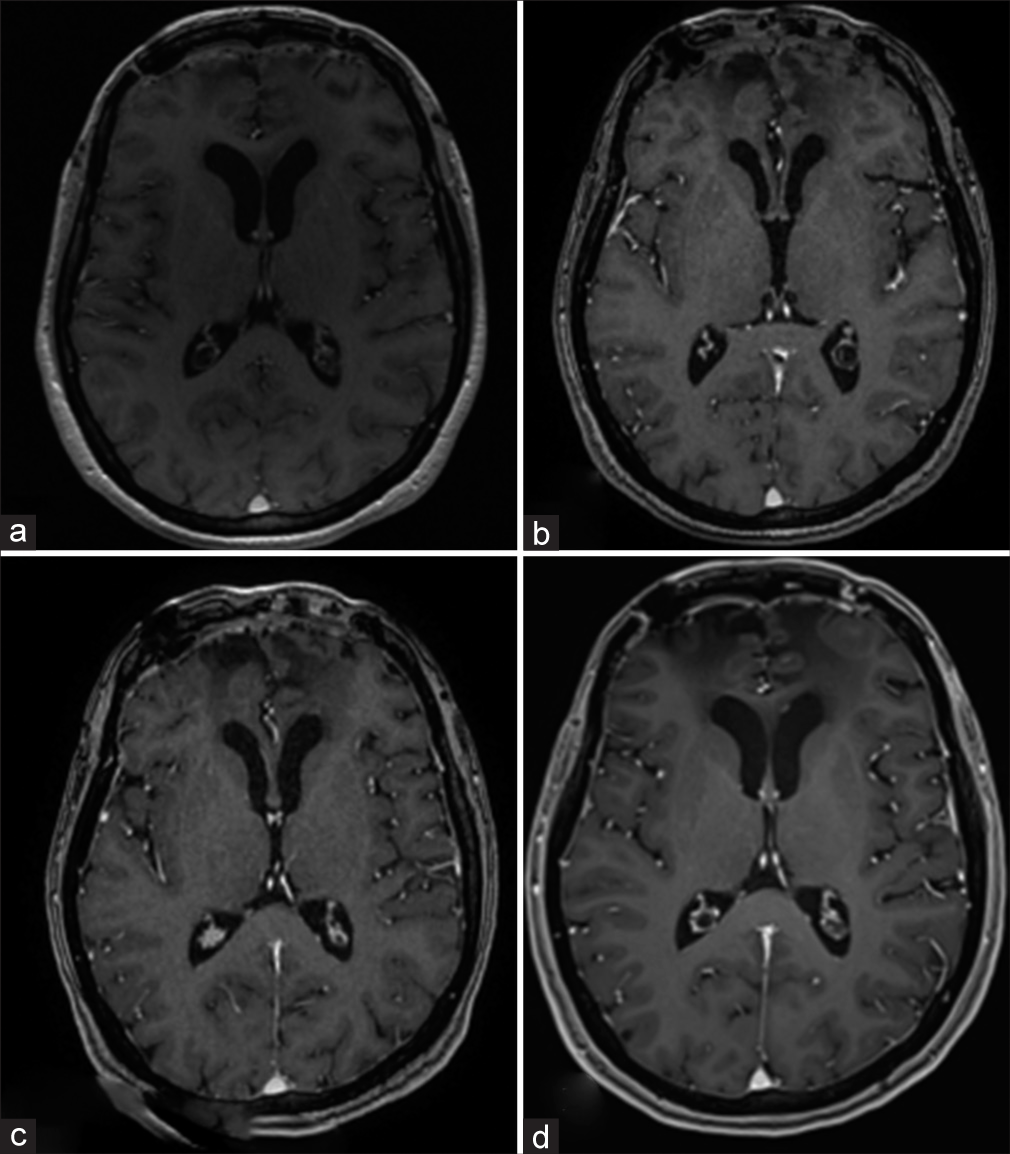
Follow-up head MRI scans were performed at seven months, 15 months, 20 months, and 31 months post-surgery [
The patient had no recurrent tumors or distant metastases nor any neurological worsening in the follow-up period of 5 years.
Case 2
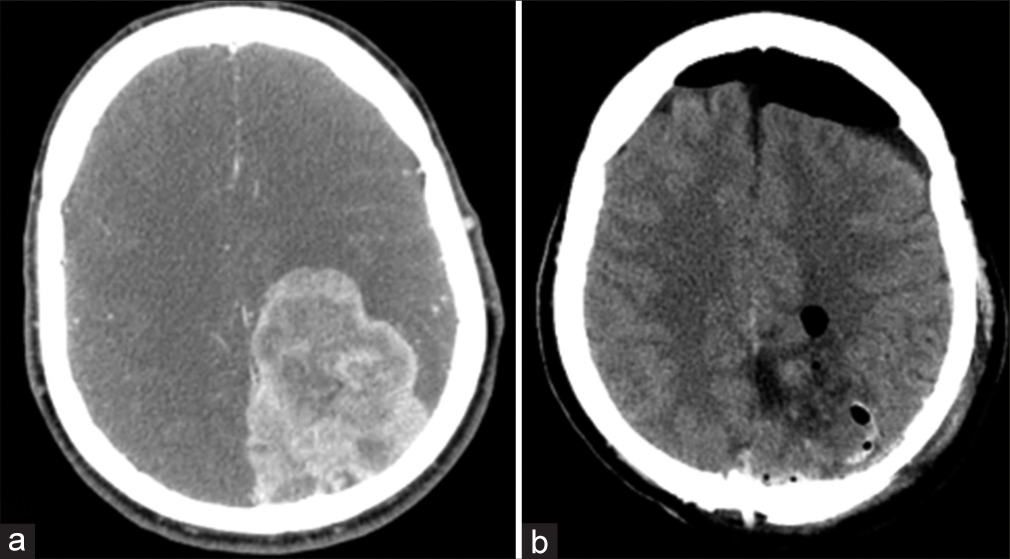
The second case is a 48-year-old male with hepatitis B who presented with sensory aphasia, right-sided hemianopsia, and 3/5 right-sided hemiparesis. CT scans revealed a large extra-axial (73 × 58 mm) parasagittal mass in the left parietooccipital region, encasing the superior sagittal sinus and dislocating the interhemispheric fissure 7 mm to the right [
He underwent a left parietal craniotomy with a preoperatively placed lumbar drain. Mannitol was applied before the durotomy and cerebrospinal fluid was drained through the lumbar drain because of the severely increased intracranial pressure. The depth and borders of the lesion were explored through the US, and the dura was opened in a C-shaped fashion based on the superior sagittal sinus. The excised tumor infiltrated the underlying cortex, the falx, and the superior sagittal sinus. Intraoperative neuromonitoring was used to verify that no functionally active zones of the motor cortex were affected. The tumor was debulked using an ultrasonic aspirator. The middle third of the superior sagittal sinus was ligated, and the infiltrated part of the falx was excised. Gross total resection was achieved. Again, intraoperative bleeding occurred, and blood loss was estimated at around 500 mL. The dura over the lesion was excised, and duraplasty was achieved with an autologous periosteal flap. Standard layered closure was performed.
Postoperatively, the patient was transferred to the intensive care unit for intensive monitoring – he was transferred back to the neurosurgical ward on postoperative day one after a head CT scan showing gross total resection of the mass [
Histopathological examination revealed a benign mesenchymal tumor with high cellularity which consisted of small monomorphic cells around vessels with HPC-like structure. Additional immunohistochemical processing showed no mitoses/10 high-power fields, as well as NSE-, Bcl2-, CD99-, CD34- and vimentin-positive and EMA-, progesterone receptor-, and CD56-negative staining. Finally, the lesion was diagnosed as a WHO grade I solitary fibrous tumor.
Six years later, the patient presented with 3/5 right-sided weakness again. CT scan showed a multifocal recurrent mass at the previous location, with the largest focus having a diameter of 49 mm [
He underwent left parasagittal recraniotomy based on the previous surgical intervention. The depth and borders of the lesion were explored through the US, and the dura was opened in a C-shaped fashion based on the superior sagittal sinus. The tumor was debulked using an ultrasonic aspirator. The excised tumor was located over the convexity – it was richly vascularized from branches of the falcine arteries. Gross total resection was achieved. Again, intraoperative bleeding occurred, and blood loss was estimated at around 500 mL. The dura was excised and duraplasty was achieved with an autologous periosteal flap. Standard layered closure was performed.
Postoperatively, the patient was transferred to the neurosurgical ward. A head CT scan was done on postoperative day 1, showing subtotal resection of the mass – a small residual tumor with a diameter of 8 mm remained on the falx [
Histopathological examination revealed a benign mesenchymal tumor with high cellularity, which consisted of cells with poor cytoplasm and large ovoid vesicular nuclei. 5 mitoses/8 mm2 were present. Again, the lesion was diagnosed as a WHO grade I solitary fibrous tumor.
DISCUSSION
SFT/M-HPC is a rare pathology which is very difficult to diagnose initially.[
Classification
The etiology of meningeal HPC is a controversial topic which has led to changes in the terminology – first, they were considered to be an angioblastic subtype of meningioma.[
Radiological findings
Meningeal HPC is very difficult to differentiate from meningiomas radiologically. CT imaging reveals both tumors as iso-dense with intense contrast enhancement, but meningiomas cause hyperostosis, while HPC may cause erosion of the overlying bone.[
MRI imaging reveals that atypical meningeal HPCs present with lobulated and/or irregular cross-leaf-shaped masses associated with prominent brain edema and frequent bone destruction, as well as a narrow base of the “dural tail.”[
Histopathological findings
Tumor size was found not to be linked to the overall survival or recurrence rate.[
Immunohistochemical staining is key for the labeling of the tumor as meningeal HPC. The 2016 WHO guidelines for the diagnosis of HPC/SFT utilize the NAB2-STAT6 fusion gene and STAT6.[
Management
Current management strategies mostly include surgery, radiotherapy, and chemotherapy.[
Contrary to some studies, Rutkowski et al. demonstrate a large-scale study which concludes that postoperative adjuvant radiotherapy does not greatly influence the overall survival rate.[
Management strategies for recurrent masses, such as our second case, are yet to be established – despite the high recurrence rate of HPC, the studies for this topic have a very limited sample size and cannot provide physicians with guidelines. However, Rutkowski et al. propose the use of surgical resection aided by adjuvant radiotherapy – their study shows that the time to second recurrence among patients who received adjuvant radiotherapy was 10.3 years compared to 5.3 years in patients who did not.[
CONCLUSION
SFT/HPC should be considered when presented with a meningioma-like tumor mass on preoperative imaging. Initial gross total resection of the tumor provides a viable management strategy, and radiotherapy may be a good option for adjuvant therapy in selected cases. Strict long-term follow-up examinations and regular MRI and/or CT scans are key to preventing the appearance of metastases and large recurrent masses, even if pathological findings show WHO grade I with low chances for recurrence and malignization, as in our second case. However, management of this pathology remains complex, and further research is necessary to establish treatment guidelines, especially regarding recurrent cases.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment section
We declare no further acknowledgments that need to be mentioned.
References
1. Bastin KT, Mehta MP. Meningeal hemangiopericytoma: Defining the role for radiation therapy. J Neurooncol. 1992. 14: 277-87
2. Chen T, Jiang B, Zheng Y, She D, Zhang H, Xing Z. Differentiating intracranial solitary fibrous tumor/hemangiopericytoma from meningioma using diffusion-weighted imaging and susceptibility-weighted imaging. Neuroradiology. 2019. 62: 175-84
3. Cohen-Inbar O. Nervous system hemangiopericytoma. Can J Neurol Sci. 2019. 47: 18-29
4. Fontaine D, Almairac F, Mondot L, Lanteri-Minet M. Cluster-like headache secondary to parasagittal hemangiopericytoma. Headache. 2013. 53: 1496-8
5. Fritchie K, Jensch K, Moskalev EA, Caron A, Jenkins S, Link M. The impact of histopathology and NAB2-STAT6 fusion subtype in classification and grading of meningeal solitary fibrous tumor/hemangiopericytoma. Acta Neuropathol. 2019. 137: 307-19
6. Haas RL, Walraven I, Lecointe-Artzner E, van Houdt WJ, Scholten AN, Strauss D. Management of meningeal solitary fibrous tumors/hemangiopericytoma; surgery alone or surgery plus postoperative radiotherapy?. Acta Oncol. 2020. 60: 35-41
7. Han Y, Zhang Q, Yu X, Han X, Wang H, Xu Y. Immunohistochemical detection of STAT6, CD34, CD99 and BCL-2 for diagnosing solitary fibrous tumors/hemangiopericytomas. Int J Clin Exp Pathol. 2015. 8: 13166-75
8. Ito H, Shoin K, Hwang WZ, Hayashi M, Yamamoto S. Meningeal hemangiopericytoma. Surg Neurol. 1986. 26: 505-11
9. Kumar N, Kumar R, Kapoor R, Ghoshal S, Kumar P, Salunke PS. Intracranial meningeal hemangiopericytoma: 10 years experience of a tertiary care Institute. Acta Neurochir. 2012. 154: 1647-51
10. Liu G, Chen ZY, Ma L, Lou X, Li SJ, Wang YL. Intracranial hemangiopericytoma: MR imaging findings and diagnostic usefulness of minimum ADC values. J Magn Reson Imaging. 2013. 38: 1146-51
11. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
12. Melone AG, D’Elia A, Santoro F, Salvati M, Delfini R, Cantore G. Intracranial hemangiopericytoma-our experience in 30 years: A series of 43 cases and review of the literature. World Neurosurg. 2014. 81: 556-62
13. Radley MG, McDonald JV. Meningeal hemangiopericytoma of the posterior fossa and thoracic spinal epidural space. Neurosurgery. 1992. 30: 446-52
14. Rutkowski MJ, Bloch O, Jian BJ, Chen C, Sughrue ME, Tihan T. Management of recurrent intracranial hemangiopericytoma. J Clin Neurosci. 2011. 18: 1500-4
15. Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T. Intracranial hemangiopericytoma. Cancer. 2011. 118: 1628-36
16. Rutkowski MJ, Sughrue ME, Kane AJ, Aranda D, Mills SA, Barani IJ. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. 2010. 113: 333-9
17. Safi SE, Godfrain J, Rooijakkers H, Collignon F. Complete resection of a torcular herophili hemangiopericytoma without sinus reconstruction: A case report and review of the literature. Case Rep Surg. 2023. 2023: 2349363
18. Shankar JJ, Hodgson L, Sinha N. Diffusion weighted imaging may help differentiate intracranial hemangiopericytoma from meningioma. J Neuroradiol. 2019. 46: 263-7
19. Winek RR, Scheithauer BW, Wick MR. Meningioma, meningeal hemangiopericytoma (angioblastic meningioma), peripheral hemangiopericytoma, and acoustic schwannoma. Am J Surg Pathol. 1989. 13: 251-61
20. Zhang Z, Li Y, She L, Wang X, Yan Z, Sun S. A footprint-like intracranial solitary fibrous tumor/hemangiopericytoma with extracranial extension and acute intratumoral hemorrhage. J Craniofac Surg. 2020. 31: e682-5