- Department of Neurosurgery, Beth Israel Deaconess Medical Center, Boston, USA
Correspondence Address:
Ekkehard M. Kasper
Department of Neurosurgery, Beth Israel Deaconess Medical Center, Boston, USA
DOI:10.4103/2152-7806.181981
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Proskynitopoulos PJ, Stippler M, Kasper EM. Post-traumatic anosmia in patients with mild traumatic brain injury (mTBI): A systematic and illustrated review. Surg Neurol Int 06-May-2016;7:
How to cite this URL: Proskynitopoulos PJ, Stippler M, Kasper EM. Post-traumatic anosmia in patients with mild traumatic brain injury (mTBI): A systematic and illustrated review. Surg Neurol Int 06-May-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/post%e2%80%91traumatic-anosmia-in-patients-with-mild-traumatic-brain-injury-mtbi-a-systematic-and-illustrated-review/
Abstract
Background:Olfactory dysfunction (OD) is a disorder associated with traumatic brain injury (TBI), which is prevalent in up to 20% of patients suffering from TBI. Nevertheless, most studies focusing on the relationship between OD and TBIs do not differentiate between the different types of TBI (mild, medium, and severe). In this paper, we conducted a comprehensive and systematic review of the existing literature for the association between mild TBI (mTBI) and OD in order to examine their relationship, focusing on its neurosurgical management and the radiographic characteristics.
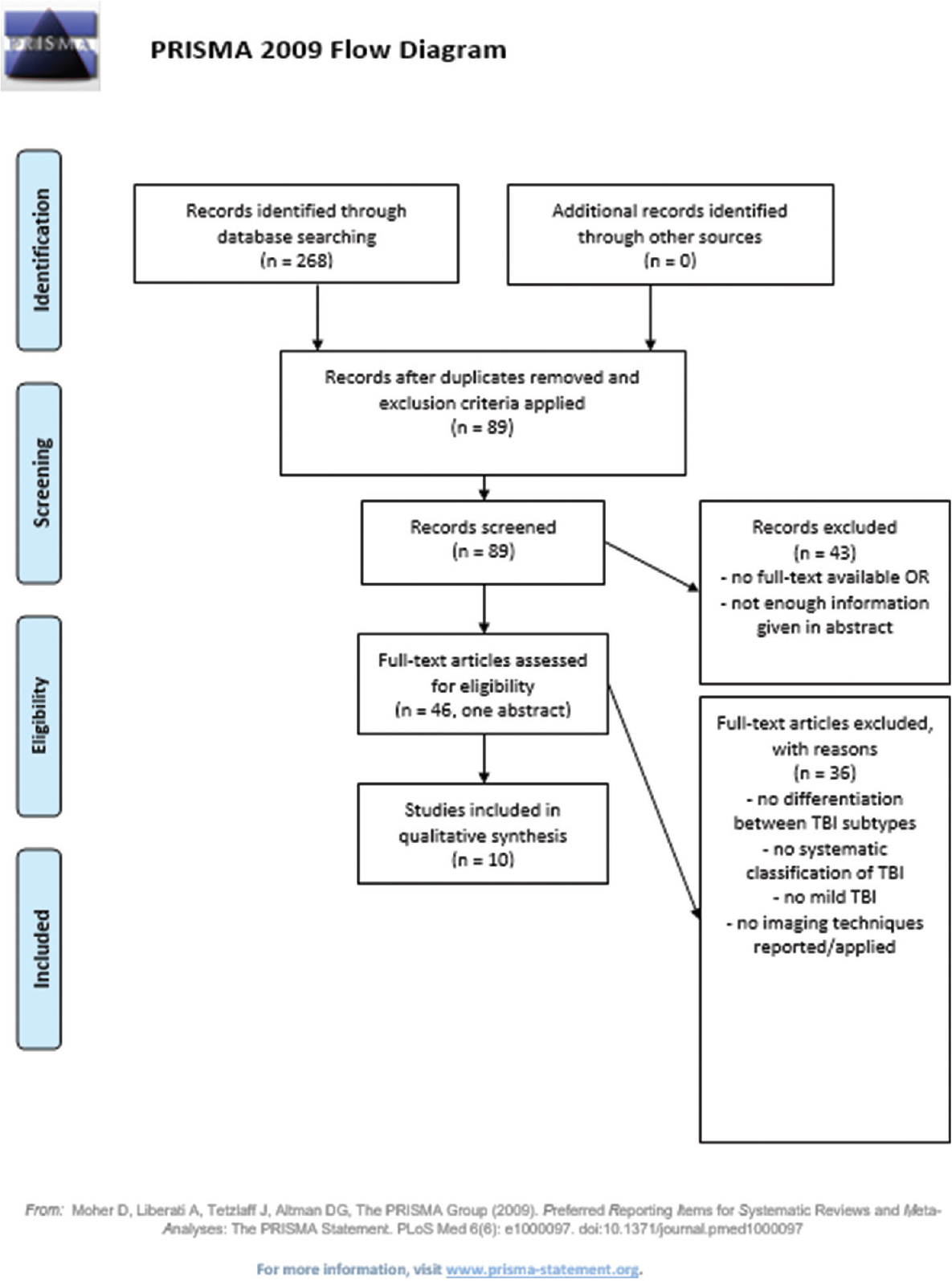
Methods:The MEDLINE database was systematically reviewed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. We found 66 articles, of which 10 fulfilled our criteria.
Results:All except two studies reported a significant association between trauma severity and olfaction. Two studies found a negative correlation between TBI severity and olfactory bulb volume with one reporting an r value of −0.62). Three studies reported an association between the observation of radiographic intracranial hemorrhage or skull base fractures and the history of TBI.
Conclusion:According to our search results, we conclude that OD is a prevalent but underdiagnosed problem in mTBI. Because OD is associated with a significant decrease in quality of life, we think that neurosurgical teams need to asses olfactory function in mTBI patients when they report to clinics. To illustrate this scenario, we include two distinct cases of patients with anosmia after mTBI in this review. Finally, we suggest a treatment algorithm for patients with mTBI so that a possible OD can be diagnosed and treated as early as possible.
Keywords: Anosmia, head trauma, olfactory dysfunction, traumatic brain injury
INTRODUCTION
Anosmia is defined as the general inability to perceive olfaction and represents the most profound olfactory dysfunction (OD). OD can be due to different pathophysiological mechanisms; it can either be a primary dysfunction and developed embryologically (e.g., due to telencephalic maldevelopment or from peripheral lesions involving the olfactory fibers at the level of the cribriform plate) or it can be due to impairment of any of the intracranial relay stations. In the latter scenarios, it may involve any of the central pathways such as the olfactory bulb (OB) and olfactory tract (OT), as well as the orbitofrontal cortex, the frontal lobe, and the antero-inferior part of the temporal lobe, the piriform cortex, the periamygdaloid cortex, the amygdala proper as well as the entorhinal cortex and the entorhinal tubercle.[
However, most frequently it is an acquired dysfunction and follows traumatic brain injury (TBI), with some studies suggesting that as many as 5% to 20% of individuals suffering from OD had sustained a preceding head trauma.[
Imaging diagnostics
Magnetic resonance imaging (MRI) studies have shown that, for TBI patients, the brain regions most frequently associated with OD are the OB and the OT, the temporal lobe, and the subfrontal lobe.[
Brain areas and functional tests associated with olfactory dysfunction
Research over the past several years has produced strong evidence that the entorhinal cortex and the OB are neuronal structures that display neuroplasticity and most importantly have the potential for significant regeneration.[
Recovery of olfactory dysfunction post-traumatic brain injury
The recovery rate of any OF depends on several clinical factors other than the anatomical location of the lesion alone. In 1995 Ikeda et al. looked at a group of patients who all had previously suffered some form of head trauma. The authors tried to examine the influence of steroids in this setting, namely bethamasone or prednisolone (30–60 mg at the beginning, followed by tapering of the dose), and examined the recovery of OF in 20 such patients.[
The relationship between trauma severity and olfactory dysfunction
Several authors have suggested that the pathophysiology of anosmia in TBI patients is correlated to the trauma severity (e.g., as defined by the Glasgow Coma Scale (GCS), the duration of Post-Traumatic Amnesia (PTA), or Loss of Consciousness (LOC)). Several studies have reported a strong association between OD and the severity of TBI.[
The purpose of our review
To the best of our knowledge, there are no reviews that have specifically examined the relationship between mTBI with its neurosurgical management as well as simultaneously examined the radiographic characteristics in patients suffering from OD. Therefore, we conducted a comprehensive review on this topic to present a coherent picture of the current state of research in this area, which we also illustrate with two distinct cases. The presented clinical vingettes will give an example of how our institution managed two exemplary patients who suffered mTBI and developed post-traumatic anosmia. Despite very similar mechanisms of injury and comparable clinical presentations, one of the patients has since then recovered her olfactory sense whereas the other patient has not. Therefore, we will try to address the following questions:
Is there evidence about an association between mTBI, OD, and certain radiographic characteristics? Why do both anosmia and OD remain a frequently underdiagnosed problem in patients suffering from mTBI? In what way could the neurosurgical management of patients suffering from mTBI be altered in order to ensure a more promising rehabilitation of patients suffering from post-traumatic OD?
CASE ILLUSTRATION #1
In 2010, a 56-year-old female reported to our clinic after being struck by a car. On arrival, the patient was amnestic to the event and could not recall the actual accident or her trip to the hospital. Her GCS was assessed as 14 and the patient was stable, alert but slightly confused, although she was oriented to herself and the date. The neurological examination showed a post-concussive syndrome, and all other investigations were without any pathological findings. No surgical intervention was necessary.
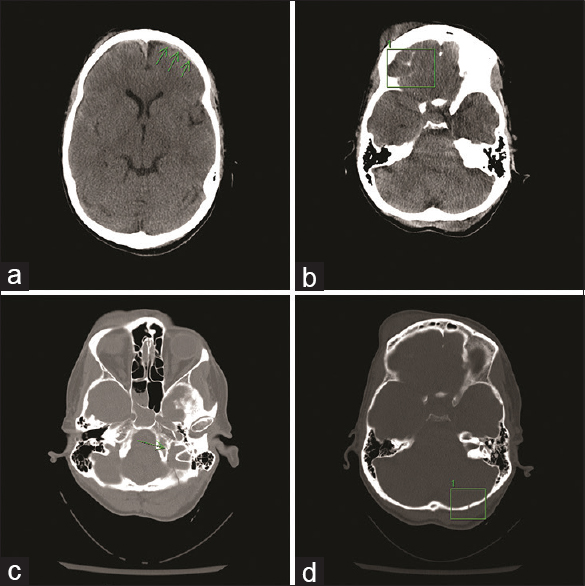
As part of her trauma and TBI work up, the on-call neurosurgeon requested a CT scan of the skull without contrast [
The patient was monitored in the intensive care unit. Head CT was repeated after 12 h to assess the intracranial hemorrhage (ICH) for potential blossoming of the contusions and expansion of the subdural haemorraghe (SDH) and subarachnoidal haemorraghe (SAH). Follow-up imaging showed no new foci of ICH. She did not develop hydrocephalus and there was no progression of ICH. She was discharged four days after her accident without any other recorded neurological abnormalities.
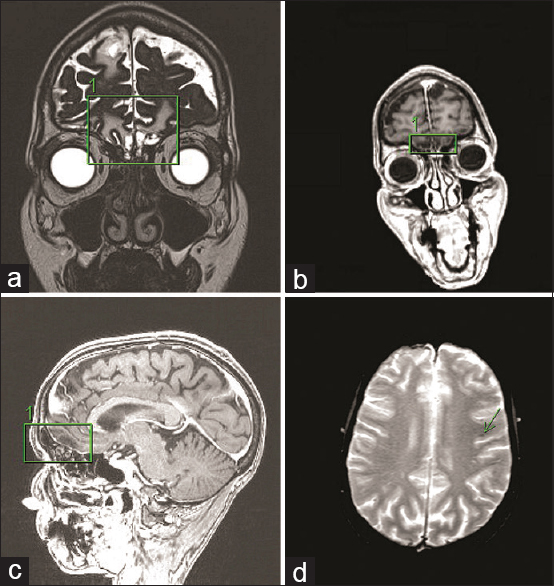
Several months later, the patient was re-evaluated in our outpatient clinic for follow up where she reported complete anosmia. No other neurological deficits were found on examination. An MRI was obtained to investigate the cause of her symptoms. No persistent or new extra-axial fluid collection or new ICH was reported. Ventricles and extra-axial cerebrospinal fluid (CSF) spaces were normal in size and configuration. MRI revealed signs of frontobasal encephalomalacia. [
Annual office visits for re-evaluation with a repeat of qualitative tests (smelling burned matches/cinnamon/coffee) for her persistent anosmia were conducted. She denied any neurological symptoms such as headache, nausea, vomiting, dizziness, or seizures or difficulty with bowel or bladder function. Examination confirmed the retained ability to taste all qualities of salty, sour, sweet, and bitter, but the patient reported a complete loss of aromatic sensations (smelling coffee, cinnamon, cloves, and food). Repeat MRI revealed no new abnormalities except the abovementioned frontobasal encephalomalacia and mild hemosiderin deposits on GRE-sequences reflecting her prior ICH. Her anosmia was explained by the mechanism of her prior head trauma resulting in a shear injury to the nerve fibers transversing the cribriform plate, the fila olfactoria. It was explained to her that regeneration of these nerve fibers and subsequent regaining of her sense of smell would be unlikely. The patient has been followed up by sequential examinations and imaging since then, and as of now, nearly five years after her TBI, she remains neurologically at her baseline without signs of functional recovery of olfaction. This case has led to complex assessments throughout the course of discovery during prolonged litigation, which most recently was settled by arbitration and has made legal history, being the first case of anosmia settled in favor of the plaintiff.[
CASE ILLUSTRATION #2
In August 2005, a 22-year-old woman reported to our clinic after being struck by a car while she was jogging. She suffered loss of consciousness at the scene but recovered to a GCS of 14 when she arrived at the hospital. When she was asked what happened, she was found to be amnestic and could not recall the actual accident or the ambulance trip.
Neurological examination upon arrival revealed that the patient was somnolent but arousable by voice. She was oriented to person but was unable to recite months or year without falling asleep. Her comprehension was good and she was able to follow commands, however, she was only speaking in short, laconic sentences. No dysarthria or paraphrasic errors were noted during the examination. All cranial nerves except the olfactory nerve were tested and no abnormalities were recorded. Her motor strength, sensation, and coordination were all found to be intact. She was assessed by orthopedic surgery for other injuries, which included fractures of her pelvis and left knee.
Following the trauma bay examination, a CT scan of the head was obtained. Imaging revealed a right skull base fracture involving the occipital bone, some minimal subarachnoid blood as well as pneumocephalus in the right posterior-fossa due to a mastoid fracture [
The patient received hydromorphone, trazodone, and ondansetron for her pain and nausea and was discharged on post-trauma day three in stable general condition with instructions to follow-up in our clinics.
Three months later, a follow-up neurological examination was performed in our outpatient clinic for reassessment. During the examination, the patient reported complete olfactory loss as her only symptom, but denied any other complaints such as headache, nausea, vomiting, dizziness, or seizures. She was also found to be neurologically intact. We disclosed to her during this clinic visit that this particular disability is most frequently observed in patients with closed head injury and associated fractures of the occipital bone. We explained to the patient that her anosmia was most likely due to a shearing of the fine nerves of the fila olfactoria and tearing at the olfactory bulb, and our advice was to consult a neurologist or an ENT specialist during follow-up in order to get a full olfactory evaluation and some suggestions for possible therapy. We concluded that no neurosurgical intervention was indicated and we would follow her expectantly only. The patient was devastated by the news of the likely permanent loss of her sense of smell as she intended to go to an esteemed culinary school in New York. The patient was later assessed at an outside facility including further radiographic studies using MRI, which showed a very minor amount of subarachnoidal hemosidering deposits from her preceding SAH but did not reveal any post-traumatic parenchymal signal changes (films not shown).
Since her injury, and after a 12-month period of complete anosmia, she has regained most of her ability to perceive aromatic scents. Her multifaceted personal reflection on this life changing experience was later expressed in an autobiographic book titled “Season to taste.”[
We were puzzled by the difference in outcome of these two individual patients, who presented with nearly identical injury patterns; hence, we set out to examine the relationship between such seemingly mTBI and its neurosurgical management and decided to simultaneously look at the radiographic characteristics of patients who were found to suffer from post-traumatic OD.
METHODS
Literature search
The review of available publications was conducted using the EBSCO Host search engine in September 2015. We reviewed MEDLINE, PSYINDEX, PsycINFO, and PsycARTICLES. We selected the following search terms and applied them to the publication abstracts: Traumatic brain injury, TBI, head trauma, post-traumatic, posttraumatic, olfactory dysfunction, olfactory impairment, anosmia, hyposmia, or olfaction disorder. These terms were combined using the Boolean algorithm terms “AND” and “OR” to retrieve pertinent study titles of reports and abstracts: (Traumatic brain injury OR TBI OR head trauma OR posttraumatic OR post-traumatic) AND (olfactory dysfunction OR olfactory impairment OR anosmia OR hyposmia OR olfaction disorder).
Among those terms, we did not include phrases such as “imaging” as we expected that some studies would not mention the use of imaging techniques in their abstracts even though they mentioned such data (which was the case for four studies). The research was done by applying the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines for systematic research [
Research criteria
The following filters were used: Abstract available, humans, English language, adults, and academic journals. We only searched the articles published between 1991 and 2015 (with the intention to cover the MRI era).
Example of full electronic search for MEDLINE so that it could be repeated
Implementation of the Boolean research term to search among titles and abstracts After the first selection, the following exclusion criteria were applied: English language, 1991 to 2015 (MRI era), adults (19+), abstracts available, and academic journals All hereby selected journals were then screened for our inclusion criteria (see below). If full text was not available, the individual abstract was screened.
After applying the exclusion criteria, we identified 89 studies, which were then fully examined for inclusion in our analysis. Inclusion criteria were: Clinical cohort studies, trials or case reports, trauma assessment, and the use of a specific evidence-based olfaction test, and report on the use of employed imaging techniques. Moreover, studies having no linked full text needed to reveal enough information in their abstract for fulfilling our criteria. Forty five available full texts and one abstract were screened. In the end ten studies (one of which was an abstract) were selected [
For assessing the risk of bias in each individual study, we restricted our review to studies using valid olfactory tests, a trauma assessment, and imaging techniques, with their respective results. To assess the risk of bias across the studies, only studies fulfilling all our inclusion criteria were selected. Therefore, we tried to minimize the risk of using unspecific data, which could not be classified to a satisfying extent, e.g., studies only mentioning that a patient had sustained a TBI but not listing the respective GCS score.
RESULTS
Forty five out of the 66 articles were available as full text via the Harvard University Countway Library access system. For the remaining studies, only abstracts were examined. In the end, ten articles fulfilled our selection criteria, and of those nine were full text articles, which are presented in
The trauma perspective
Within the identified ten studies, a total of 350 patients were described as having sustained mTBI. For classification purposes, all three TBI indicators, namely GCS,[
Two studies reported an association between OD and PTA.[
A neurological point of view in respect to the used olfactory tests
Identified studies were then reviewed in detail, and we found that overall eight different olfactory tests were used. Three studies[
Radiographic analysis
Eight of the ten identified studies reported the use of MRI for imaging, while six studies additionally used CT scanning and one study employed single-photon emission computed tomography (SPECT) imaging in combination with MRI. Reported abnormalities included hemorrhages,[
Three studies found an association between the observation of post-traumatic radiographic intracranial hemorrhage[
Follow up observations
Six studies[
DISCUSSION
Post-traumatic anosmia or olfactory dysfunction is a prevalent but surprisingly under-evaluated[
To get a clear view about the current status of the field, we conducted a review of the pertinent literature, which addresses this issue from three different viewpoints; a trauma perspective, a neurological viewpoint, and a radiographic analysis of the patient population. This review revealed that there are no available prospective studies and that the only established evidence is obtained from retrospective cohort studies, which report rather heterogeneous information concerning anosmia or OD, in general, for mTBI patients. One explanation for the lack of a better scientific understanding and the lack of more concrete evidence for the association between mTBI and OD is the fact that many trauma centers do not assess this problem systematically or with any quantifiable tests and longitudinal follow-up. Furthermore, they do not employ established scales (e.g., LOC, PTA, and GCS) in order to differentiate between the different types of TBI. From a neurosurgical point of view, this creates a problem of validity in predicting outcome and in selecting and recommending any possible rehabilitative interventions because significant differences exist between the different TBI types and their prognosis. Therefore, we want to stress that tools of proper patient classification need to be employed strictly and consistently and also need to be repeated thoroughly during longitudinal follow-up.
The association between trauma and olfactory dysfunction
A possible reason for the lack of differentiation in the trauma aspect of such studies may be the fact that most of the retrieved studies were not published by neurosurgeons but by post-hoc care providers such as otorhinolaryngologists and neuropsychologists. Still, for the few OD studies that did contain information about neurosurgical trauma severity (allowing the identification of mTBI), an association of OD with different radiographic lesion types could be observed. In addition to this, a significant difference in the post-traumatic level of OF correlating to the injury severity could be demonstrated. This showed that, even in patients with mTBIs, such trauma can lead to structural impairment and radiographically distinct “pathologies” that correlate with OD.
This is in contrast to some conclusions drawn by a review published earlier this year.[
Imaging techniques
Schofield and colleagues note, in their review, that anosmia in mTBI patients is often associated with more severe radiographic pathologies (such as hemorrhages and contusions). They also mentioned that a diagnosis of anosmia may also be suggested by certain behavioral changes. This raises the point that a high index of suspicion needs to be developed by healthcare providers, which could be based on anamnesis and mechanism of injury, and emphasizes how important it is to assess OD in the early stages.[
Second, most current trauma guidelines advise against the use of steroids in brain trauma patients, even though the prescription of steroids was one of the few interventions in OD patients, which was shown to have a positive effect on the regeneration of OF. Beyond this, there is evidence in the literature suggesting that olfactory training mechanisms with different odor discrimination tests would probably also help in rehabilitation of patients with OD.
Aspects of rehabilitation
One of the pressing questions that needs to be raised here is whether head injury teams (trauma or neurosurgical) should routinely assess OF in post-TBI patients who report to a clinic, and if so when. It is certainly true that, in cases of moderate or severe TBI, immediate neurosurgical management has other priorities than to assess the patients’ OF in the acute setting, especially if the patient is in a very critical state. However, for any patient who presents with mild head trauma, a diagnosis of OD can make a difference in planning further care and coping strategies and can also lead to interventions which might yield recovery of some function via possible rehabilitation. To this end, one should keep in mind that the diagnosis of OD, though belittled by many in the field, has a severe effect on each patient's QOL.
Several studies have shown such profound effect of OD on QOL. For example, due to the inability in identifying and discriminating between odors, anosmic patients suffer significantly higher risks of hazardous events such as those due to gas leaks and fires in general.[
When looking at the results presented here, we must bear in mind some methodical strains, which need to be explained further; because of the various olfactory tests and TBI classifications used in the available literature, the few available studies dealing with this topic cannot be compared easily. Our review has shown that currently published data are not very strong in establishing a clear and unequivocal association between mTBI and OD. As we have pointed out above, some studies were able to demonstrate such a correlation but one failed to show a significant difference between a group of mTBI patients and the control group.[
Management of mTBI patients suffering from anosmia
Our results and the theoretical background presented have shown that both an association between mTBI and radiographic images as well as a possible treatment procedure exists. However, the current management guidelines make the line of argument complicated because it is well-known that MRI is superior to CT scanning for many disease entities and this has been corroborated in patients suffering from concussions in general. In this patient population, pathological changes are visible in as many as 25% of the examined cases despite the fact that initial CT was normal.[
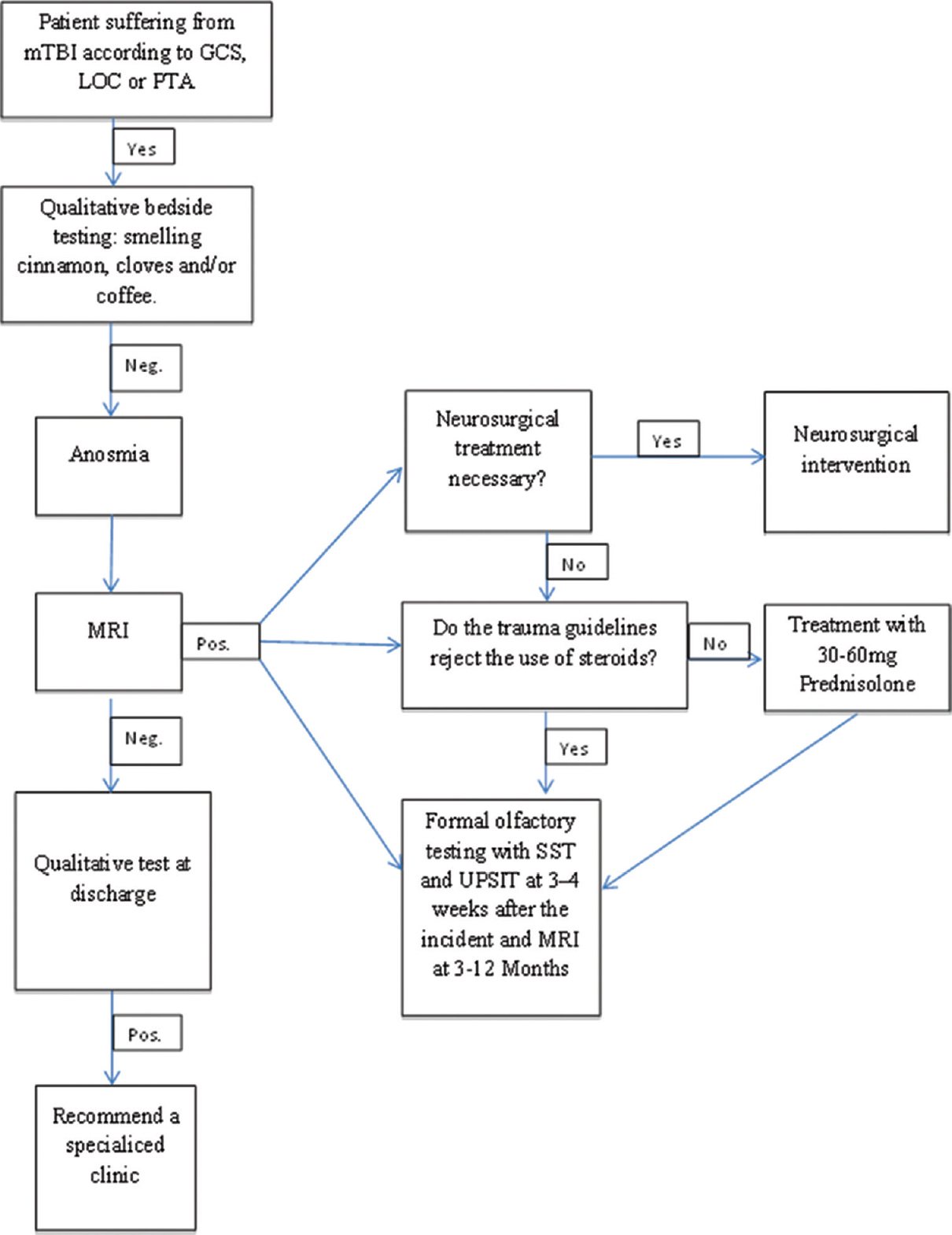
A possible treatment algorithm: Summary of the evidence found
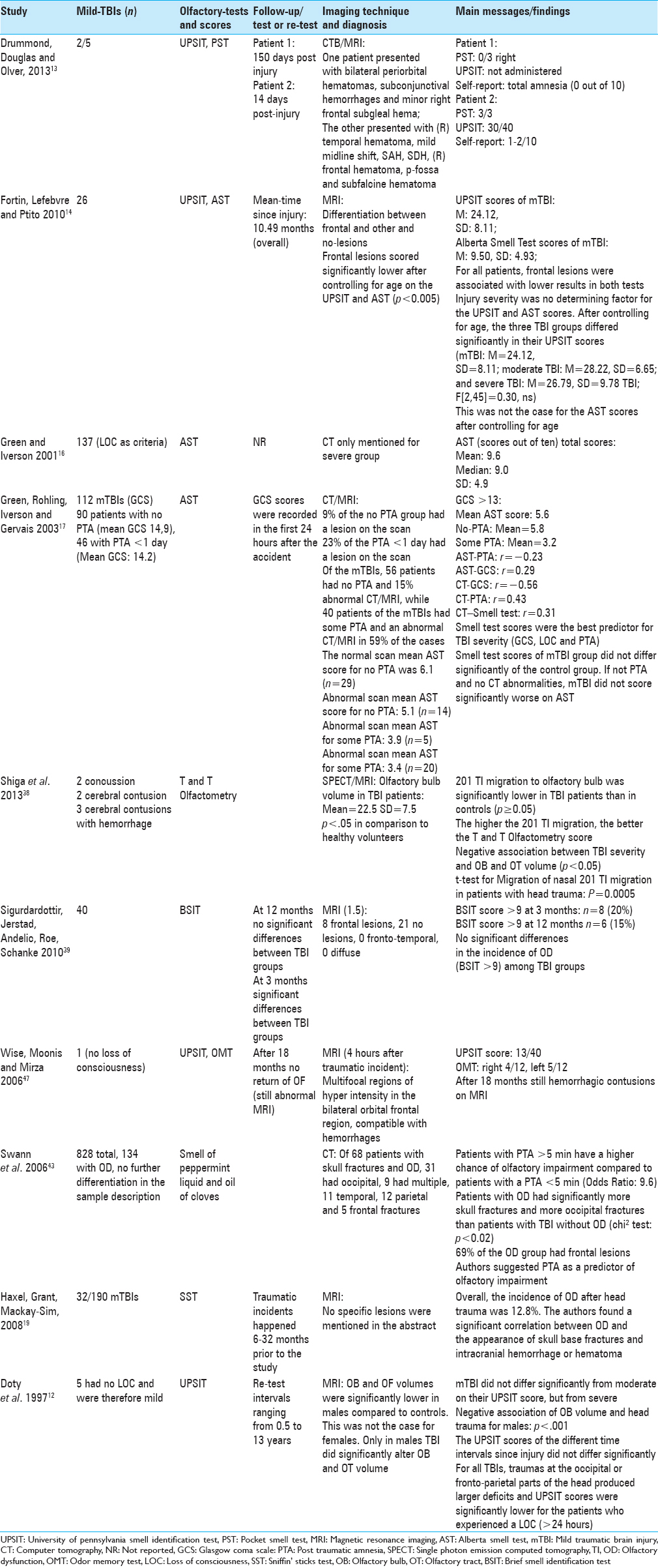
Based on the theoretical background presented above and the results of our review, we would suggest a treatment algorithm [
Figure 4
Treatment algorithm for patients who report to a clinic with mTBI. Depending on the diagnosis of a mTBI, in combination with an olfactory dysfunction, we suggest the described scheme that should be followed in order to manage the patient accordingly. Abbreviations: mTBI: mild traumatic brain injury, GCS: Glasgow Coma Scale, LOC: Loss of Consciousness, SST: Scratch and Sniff Test, UPSIT; University of Pennsylvania Smell Identification Test
Along with this rather simple scheme, the trauma team or neurosurgeon could send the patient to a specialized OD clinic in order to better evaluate such patients with tests such as the SST. Should this result in any recovery of OF following the trauma, the patients’ QOL will probably be improved or sustained. Therefore, for future research, we recommend conducting a prospective study in order to investigate whether patients with post-traumatic OD from mTBI show better functional recovery and better QOL scores than patients who do not receive specific OF-related interventions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alderfer BS, Arciniegas DB, Silver JM. Treatment of depression following traumatic brain injury. J Head Trauma Rehabil. 2005. 20: 544-62
2. Anstey KJ, Butterworth P, Jorm AF, Christensen H, Rodgers B, Windsor TD. A population survey found an association between self-reports of traumatic brain injury and increased psychiatric symptoms. J Clin Epidemiol. 2004. 57: 1202-9
3. Atighechi S, Salari H, Baradarantar MH, Jafari R, Karimi G, Mirjali M. A comparative study of brain perfusion single-photon emission computed tomography and magnetic resonance imaging in patients with post-traumatic anosmia. Am J Rhinol Allergy. 2009. 23: 409-12
4. Atighechi S, Zolfaghari A, Baradaranfar M, Dadgarnia M. Estimation of sensitivity and specificity of brain magnetic resonance imaging and single photon emission computed tomography in the diagnosis of olfactory dysfunction after head traumas. Am J Rhinol Allergy. 2013. 27: 403-6
5. Birnbaum M.editors. Season to Taste. New York: Harper Collins; 2011. p.
6. Buschhuter D, Smitka M, Puschmann S, Gerber JC, Witt M, Abolmaali ND. Correlation between olfactory bulb volume and olfactory function. NeuroImage. 2008. 42: 498-502
7. Costanzo RM. Neural regeneration and functional reconnection following olfactory nerve transection in hamster. Brain Res. 1985. 361: 258-66
8. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life--an updated review. Chem Senses. 2014. 39: 185-94
9. Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991. 117: 519-28
10. Doty RL. Office procedures for quantitative assessment of olfactory function. Am J Rhinol. 2007. 21: 460-73
11. Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys. 1989. 45: 381-4
12. Doty RL, Yousem DM, Pham LT, Kreshak AA, Geckle R, Lee WW. Olfactory dysfunction in patients with head trauma. Arch Neurol. 1997. 54: 1131-40
13. Drummond M, Douglas J, Olver J. ’If I haven’t got any smell…I’m out of work’: Consequences of olfactory impairment following traumatic brain injury. Brain Inj. 2013. 27: 332-45
14. Fortin A, Lefebvre MB, Ptito M. Traumatic brain injury and olfactory deficits: The tale of two smell tests!. Brain Inj. 2010. 24: 27-33
15. Fujii M, Fukazawa K, Takayasu S, Sakagami M. Olfactory dysfunction in patients with head trauma. Auris, Nasus, Larynx. 2002. 29: 35-40
16. Green P, Iverson GL. Effects of injury severity and cognitive exaggeration on olfactory deficits in head injury compensation claims. NeuroRehabilitation. 2001. 16: 237-43
17. Green P, Rohling ML, Iverson GL, Gervais RO. Relationships between olfactory discrimination and head injury severity. Brain Inj. 2003. 17: 479-96
18. Haehner A, Rodewald A, Gerber JC, Hummel T. Correlation of olfactory function with changes in the volume of the human olfactory bulb. Arch Otolaryngol Head Neck Surg. 2008. 134: 621-4
19. Haxel BR, Grant L, Mackay-Sim A. Olfactory dysfunction after head injury. J Head Trauma Rehabil. 2008. 23: 407-13
20. Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007. 264: 237-43
21. Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005. 125: 116-21
22. Hummel T, Rissom K, Reden J, Hahner A, Weidenbecher M, Hüttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009. 119: 496-9
23. Ikeda K, Sakurada T, Takasaka T, Okitsu T, Yoshida S. Anosmia following head trauma: Preliminary study of steroid treatment. Tohoku J Exp Med. 1995. 177: 343-51
24. Jagoda AS, Bazarian JJ, Bruns JJ, Cantrill SV, Gean AD, Howard PK. Clinical policy: Neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008. 52: 714-48
25. Jiang RS, Chai JW, Chen WH, Fuh WB, Chiang CM, Chen CC. Olfactory bulb volume in Taiwanese patients with posttraumatic anosmia. Am J Rhinol Allergy. 2009. 23: 582-4
26. Jiang RS, Wu SH, Liang KL, Shiao JY, Hsin CH, Su MC. Steroid treatment of posttraumatic anosmia. Eur Arch Otorhinolaryngology. 2010. 267: 1563-7
27. Katotomichelakis M, Simopoulos E, Tripsianis G, Prokopakis E, Danielides G, Velegrakis SG. Improvement of olfactory function for quality of life recovery. Laryngoscope. 2013. 123: E10-6
28. Kern RC, Quinn B, Rosseau G, Farbman AI. Post-traumatic olfactory dysfunction. Laryngoscope. 2000. 110: 21-9
29. Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013. 123: E85-90
30. Levin HS, Williams DH, Eisenberg HM, High WM, Guinto FC. Serial MRI and neurobehavioural findings after mild to moderate closed head injury. J Neurol Neurosurg Psychiatry. 1992. 55: 255-62
31. Mueller CA, Hummel T. Recovery of olfactory function after nine years of post-traumatic anosmia: A case report. J Med Case Rep. 2009. 3: 9283-
32. Nguyen AD, Pelavin PE, Shenton ME, Chilakamarri P, McCarley RW, Nestor PG. Olfactory sulcal depth and olfactory bulb volume in patients with schizophrenia: An MRI study. Brain Imaging Behav. 2011. 5: 252-61
33. Rombaux P, Bertrand B, Keller T, Mouraux A. Clinical significance of olfactory event-related potentials related to orthonasal and retronasal olfactory testing. Laryngoscope. 2007. 117: 1096-101
34. Rombaux P, Duprez T, Hummel T. Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology. 2009. 47: 3-9
35. Royet JP, Croisile B, Williamson-Vasta R, Hibert O, Serclerat D, Guerin J. Rating of different olfactory judgements in Alzheimer's disease. Chem senses. 2001. 26: 409-17
36. Santos DV, Reiter ER, DiNardo LJ, Costanzo RM. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004. 130: 317-9
37. Schofield PW, Moore TM, Gardner A. Traumatic brain injury and olfaction: A systematic review. Front Neurolo. 2014. 5: 5-
38. Shiga H, Taki J, Washiyama K, Yamamoto J, Kinase S, Okuda K. Assessment of olfactory nerve by SPECT-MRI image with nasal thallium-201 administration in patients with olfactory impairments in comparison to healthy volunteers. Plos One. 2013. 8: e57671-
39. Sigurdardottir S, Jerstad T, Andelic N, Roe C, Schanke AK. Olfactory dysfunction, gambling task performance and intracranial lesions after traumatic brain injury. Neuropsychology. 2010. 24: 504-13
40. Simopoulos E, Katotomichelakis M, Gouveris H, Tripsianis G, Livaditis M, Danielides V. Olfaction-associated quality of life in chronic rhinosinusitis: Adaptation and validation of an olfaction-specific questionnaire. Laryngoscope. 2012. 122: 1450-4
41. Staff MLW. Pedestrian struck by car loses sense of smell, taste. Massachusetts Lawyers Weekly. 2015. p.
42. Stiell IG, Clement CM, Rowe BH, Schull MJ, Brison R, Cass D. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005. 294: 1511-8
43. Swann IJ, Bauza-Rodriguez B, Currans R, Riley J, Shukla V. The significance of post-traumatic amnesia as a risk factor in the development of olfactory dysfunction following head injury. Emerg Med J. 2006. 23: 618-21
44. Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000. 157: 828-30
45. Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008. 255: 1121-6
46. Wang L, Chen L, Jacob T. Evidence for peripheral plasticity in human odour response. J Physiol. 2004. 554: 236-44
47. Wise JB, Moonis G, Mirza N. Magnetic resonance imaging findings in the evaluation of traumatic anosmia. Ann Otol Rhinol Laryngol. 2006. 115: 124-7
48. Yee KK, Costanzo RM. Restoration of olfactory mediated behavior after olfactory bulb deafferentation. Physiol Behav. 1995. 58: 959-68
49. Yousem DM, Geckle RJ, Bilker WB, Kroger H, Doty RL. Posttraumatic smell loss: Relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad Radiol. 1999. 6: 264-72
50. Yousem DM, Geckle RJ, Bilker WB, McKeown DA, Doty RL. Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR Am J Neuroradiol. 1996. 17: 1171-9