- Department of Neurosurgery, Tohoku University, Graduate School of Medicine, Sendai, Japan
- Department of Neurosurgery, Mansoura University, Mansoura, Egypt
- Department of Neurosurgery, Kohnan Hospital, Sendai, Japan
- Department of Neurosurgery, Sendai Medical Center, Sendai, Japan
Correspondence Address:
Toshiki Endo
Department of Neurosurgery, Tohoku University, Graduate School of Medicine, Sendai, Japan
DOI:10.4103/2152-7806.195585
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hosam Shata Mohanmed Ali, Toshiki Endo, Hidenori Endo, Kensuke Murakami, Teiji Tominaga. Intraspinal dissemination of intracranial hemangiopericytoma: Case report and literature review. 12-Dec-2016;7:
How to cite this URL: Hosam Shata Mohanmed Ali, Toshiki Endo, Hidenori Endo, Kensuke Murakami, Teiji Tominaga. Intraspinal dissemination of intracranial hemangiopericytoma: Case report and literature review. 12-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/intraspinal-dissemination-of-intracranial-hemangiopericytoma-case-report-and-literature-review/
Abstract
Background:The authors report the case of a 53-year-old woman suffering from thoracic myelopathy caused by intraspinal dissemination of hemangiopericytoma. In literature, hemangiopericytoma is commonly found as an intracranial lesion, and often hematogenously metastasizes to the bone or liver; however, intradural spinal dissemination is extremely rare.
Case Description:The patient presented with gait disturbance due to thoracic myelopathy 6 years after surgical treatment for intracranial hemangiopericytoma. Magnetic resonance imaging demonstrated intradural disseminated lesions compressing the spinal cord. Although the patient underwent resection of the intradural spinal tumor, the lesion was tightly adherent to the dorsal surface of the spinal cord. Therefore, it resulted in subtotal removal. Immediately after the surgery, symptoms related to the thoracic myelopathy resolved. The patient was free from disease progression for 14 months after whole spine radiotherapy.
Conclusion:Recognition of this type of progression is important in the clinical management of intracranial hemangiopericytoma because intradural spinal dissemination dramatically degrades neurological functions.
Keywords: Hemangiopericytoma, myelopathy, spinal dissemination
INTRODUCTION
Hemangiopericytoma is a neoplasm that commonly manifests as an intracranial mass lesion.[
Here, we report a case of intracranial hemangiopericytoma in which recurrence manifested as intraspinal dissemination. To our knowledge, subarachnoid spread via intraspinal dissemination has not been previously reported in English literature. It is important to recognize this rare form of disease progression to achieve better control in hemangiopericytoma.
CASE REPORT
Clinical history
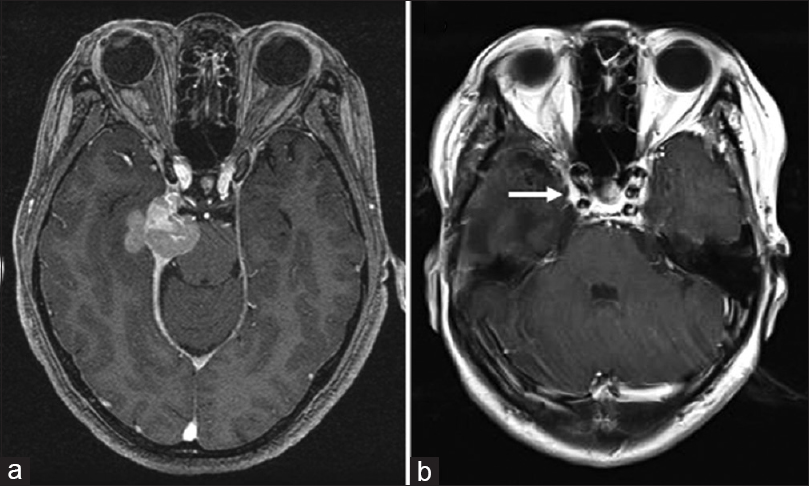
In 2007, a 53-year-old woman was referred to our department with a chief complaint of double vision. She had an intracranial tumor originating from her right tentorium and underwent subtotal surgical resection [
Figure 1
Magnetic resonance images from the original patient presentation in 2007. (a) Preoperatively, axial T1-weighted contrast images demonstrated a mass lesion located along the right tentorium, extending toward the temporal lobe and the pons. (b) Postoperative T1-weighted contrast image. A tumor invading into the cavernous sinus (arrow) remained. The extent of resection was judged as “subtotal”
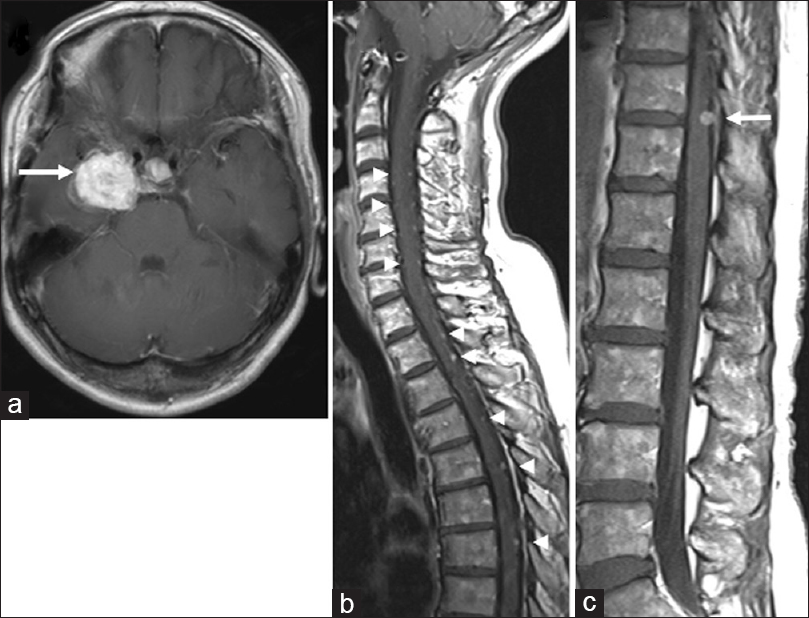
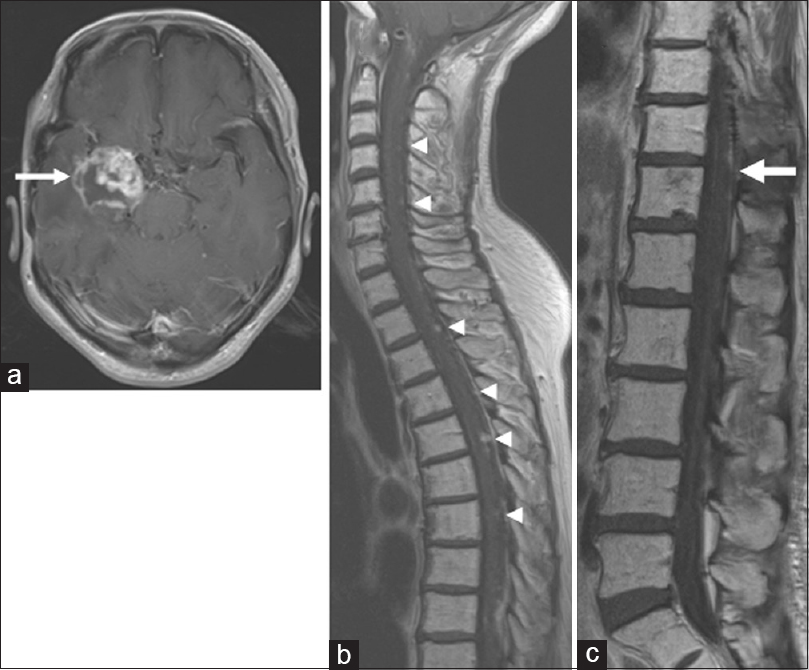
In 2013, the patient was referred back to us with a recurrent intracranial lesion [
Figure 2
Magnetic resonance images from 2013 when the patient was referred back to us with a recurrent intracranial lesion. (a) Axial T1-weighted image of the brain, demonstrating a recurrent mass lesion (arrow). (b, c) Sagittal T1-weighted images demonstrating spinal disseminated lesions (arrowheads). The tumor at T11/12 (arrow) did not cause thoracic myelopathy at that time
Clinical course and examination
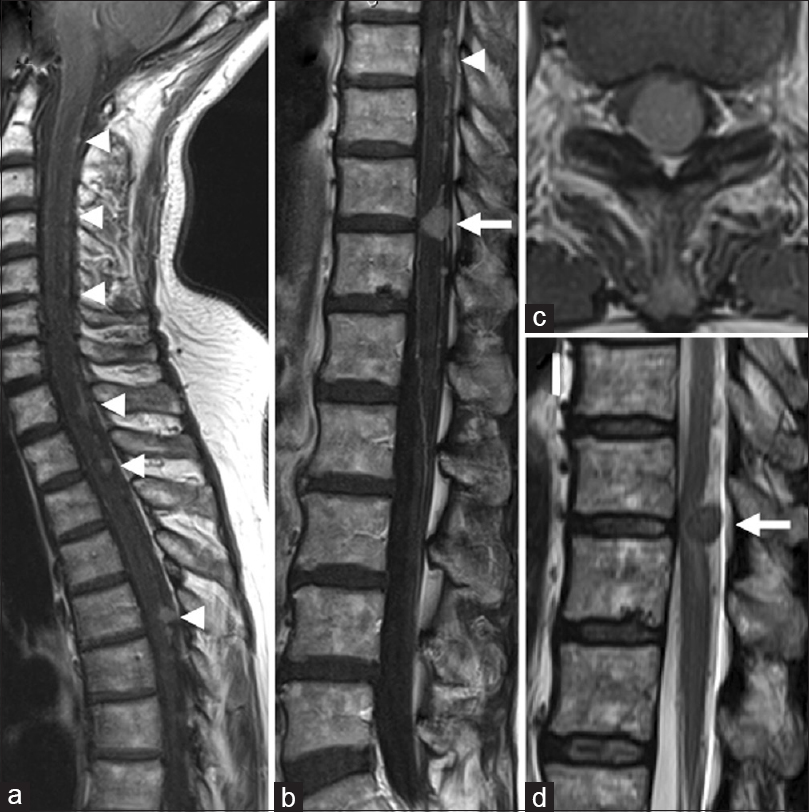
There was no further growth of the intracranial recurrent tumor. Six months after the radiosurgery, she acutely developed gait disturbance. Neurological examination showed left lower limb weakness with bilaterally increased patellar and Achilles tendon reflexes. Sensory examination revealed decreased superficial and deep sensations below the T12 dermatome. MRI demonstrated enlargement of multiple intraspinal lesions spanning the spinal cord [
Figure 3
Preoperative magnetic resonance images of the spinal cord. (a, b) Sagittal T1-weighted images with gadolinium, demonstrating intraspinal disseminated lesions that were increased in size (arrowheads). Note the T11/12 lesion (arrow in B) compressing the spinal cord. (c) Axial T1-weighted contrast image demonstrating the T11/12 tumor now causing a deformity of the spinal cord. (d) Sagittal T2-weighted image demonstrating an abnormal hyperintense area in the dorsal spinal cord where the T11/12 lesion is located (arrow)
Surgical interventions
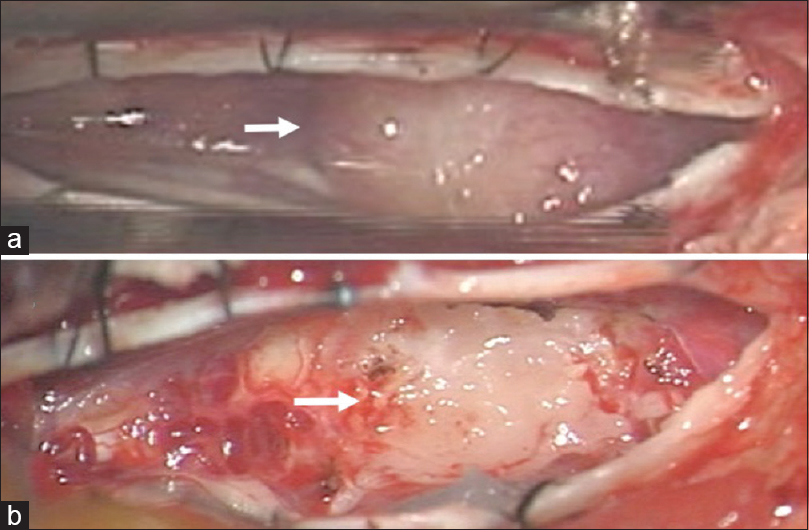
The patient experienced aggravated symptoms related to thoracic myelopathy and agreed to undergo surgical resection of the T11 lesion. Under monitoring of motor and sensory evoked potentials, the patient was placed in the prone position. Following a left T11 hemilaminectomy, the tumor was exposed. Importantly, the tumor was located in the subarachnoid space and was tightly adherent to the spinal cord surface [
Figure 4
Intraoperative photographs following left T11 hemilaminectomy. (a) Following the dural opening, a mass lesion was visible underneath the arachnoid membrane (arrow). (b) Subtotal surgical resection was performed. A layer of the tumor was left attached to the dorsal spinal cord (arrow). Note that the left and right sides of the images were the rostral and caudal sides, respectively
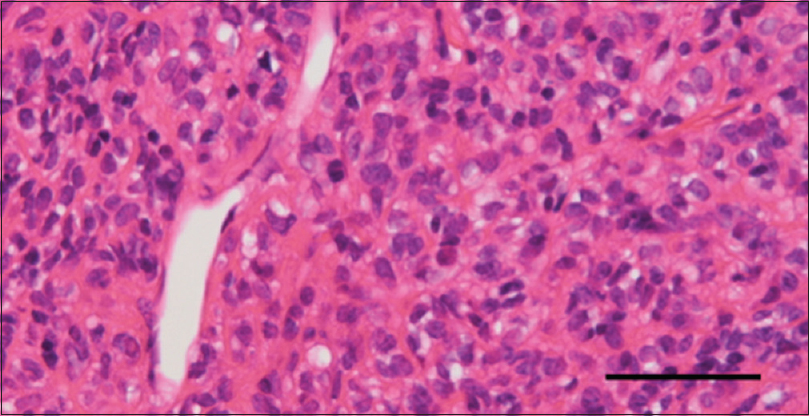
Pathological diagnosis
Pathological evaluations demonstrated a hypercellular tumor with numerous slit-like vascular channels called staghorn sinusoids [
Postoperative course
Immediately after the surgery, the symptoms due to the thoracic myelopathy improved. A month after the surgery, the patient did not have motor weakness. Her sensory symptoms were also resolved. To prevent other spinal lesions from becoming symptomatic, radiotherapy covering the whole spinal cord was performed (50.4 Gy). At the last follow-up, 16 and 14 months after spinal tumor removal and intraspinal radiotherapy, respectively, the patient was free from new symptoms or recurrences. MRI confirmed that the remnant lesions were stable in size [
Figure 6
Follow-up magnetic resonance images 16 months after resection. (a) Axial T1-weighted image of the brain, demonstrating an intracranial mass lesion (arrow) that was stable in size. Note that it had an altered intensity following the gamma knife treatment. (b, c) Sagittal T1-weighted images demonstrating spinal disseminated lesions (arrowheads) that were stable in size. The resected spinal tumor (arrow) did not recur
DISCUSSION
Intracranial hemangiopericytoma is often complicated by extracranial metastasis. A large clinical series on intracranial hemangiopericytomas reported the rates of extracranial metastasis as 20–55%.[
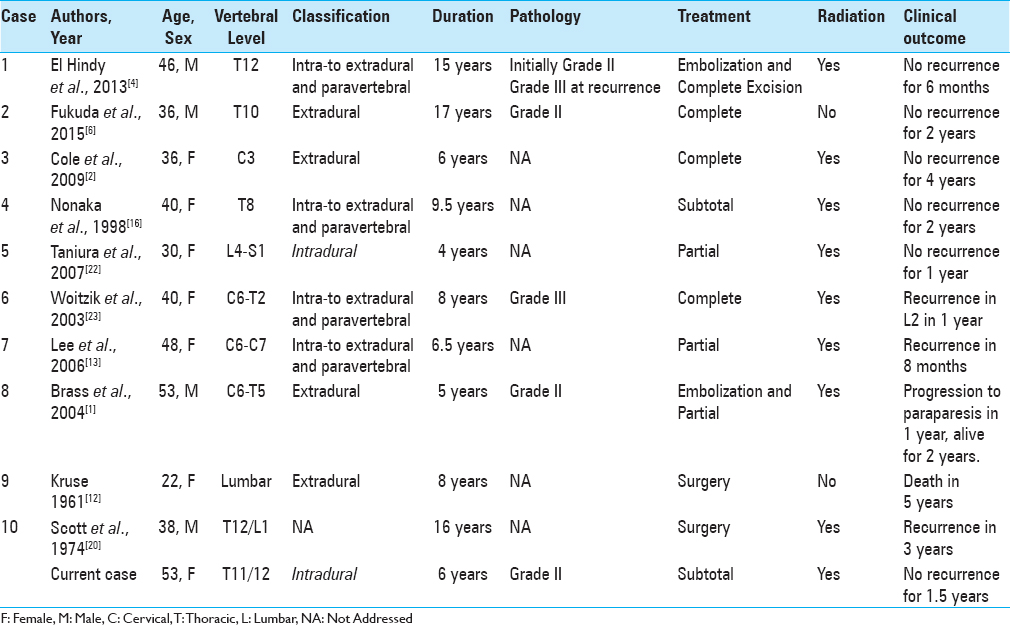
We have summarized 10 previously reported cases of metastatic spinal hemangiopericytoma in
Metastasis usually occurs late, well after the diagnosis of the primary lesion. Guthrie et al. reported 99 months as the average time to metastasis.[
To date, clear predisposing factors for metastatic hemangiopericytoma have not been established. However, the mechanisms or predisposing factors leading to drop metastasis in different intracranial pathologies besides hemangiopericytoma have been discussed. In glioblastoma, Shibahara et al. reported that the higher expression of CD133, a stem cell marker, correlated with an increased incidence of distant metastasis. In oligodendroglioma, the proximity to cisterns and ventricles facilitated the spread through the CSF.[
In hemangiopericytoma, a size of ≥6 cm and a non-skull base location were found to be predictive of local recurrences.[
Comparing the grades of hemangiopericytoma,[
There is no consensus on the best treatment for metastatic hemangiopericytoma. When considering surgical resection, the high vascularity of this disease should be taken into account.[
CONCLUSIONS
Intracranial hemangiopericytoma can cause intraspinal dissemination. This rare form of disease progression should be recognized for better therapeutic management.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank Enago for English review.
References
1. Brass SD, Guiot MC, Albrecht S, Glikstein R, Mohr G. Metastatic hemangiopericytoma presenting as an epidural spinal cord lesion. Can J Neurol Sci. 2004. 31: 550-3
2. Cole CD, Schmidt MH. Hemangiopericytomas of the spine: Case report and review of the literature. Rare Tumors. 2009. 1: e43-
3. Damodaran O, Robbins P, Knuckey N, Bynevelt M, Wong G, Lee G. Primary intracranial haemangiopericytoma: Comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci. 2014. 21: 1310-4
4. El Hindy N, Ringelstein A, Forsting M, Sure U, Mueller O. Spinal metastasis from malignant meningeal intracranial hemangiopericytoma: One-staged percutaneous Onyx embolization and resection-a technical innovation. World J Surg Oncol. 2013. 11: 152-
5. Elefante A, Peca C, Del Basso De Caro ML, Russo C, Formicola F, Mariniello G. Symptomatic spinal cord metastasis from cerebral oligodendroglioma. Neurol Sci. 2012. 33: 609-13
6. Fukuda Y, Watanabe K, Toyama Y, Mikami S, Matsumoto M. Metastasis of intracranial meningeal hemangiopericytoma to thoracic spine 17 years after surgical excision: A case report. J Orthop Sci. 2015. 20: 425-9
7. Ghia AJ, Chang EL, Allen PK, Mahajan A, Penas-Prado M, McCutcheon IE. Intracranial hemangiopericytoma: Patterns of failure and the role of radiation therapy. Neurosurgery. 2013. 73: 624-31
8. Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma: Histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989. 25: 514-22
9. Jaaskelainen J, Servo A, Haltia M, Wahlstrom T, Valtonen S. Intracranial hemangiopericytoma: Radiology, surgery, radiotherapy, and outcome in 21 patients. Surg Neurol. 1985. 23: 227-36
10. Johnson DR, Kimmel DW, Burch PA, Cascino TL, Giannini C, Wu W. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. 2011. 13: 530-5
11. Kim JH, Jung HW, Kim YS, Kim CJ, Hwang SK, Paek SH. Meningeal hemangiopericytomas: Long-term outcome and biological behavior. Surg Neurol. 2003. 59: 47-54
12. Kruse F. Hemangiopericytoma of the meniges (angioblastic meningioma of Cushing and Eisenhardt). Clinico-pathologic aspects and follow-up studies in 8 cases. Neurology. 1961. 11: 771-7
13. Lee JK, Kim SH, Joo SP, Kim TS, Jung S, Kim JH. Spinal metastasis from cranial meningeal hemangiopericytomas. Acta Neurochir. 2006. 148: 787-90
14. Liu HG, Yang AC, Chen N, Yang J, Qiu XG, Zhang JG. Hemangiopericytomas in the spine: Clinical features, classification, treatment, and long-term follow-up in 26 patients. Neurosurgery. 2013. 72: 16-24
15. Lorigan P, Verweij J, Papai Z, Rodenhuis S, Le Cesne A, Leahy MG. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 2007. 25: 3144-50
16. Nonaka M, Kohmura E, Hirata M, Hayakawa T. Metastatic meningeal hemangiopericytoma of thoracic spine. Clin Neurol Neurosurg. 1998. 100: 228-30
17. Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A, Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.editors. Meningeal tumours. WHO classification of tumours of the central nervous system. Lyon: IARC; 2007. p. 164-72
18. Rutkowski MJ, Bloch O, Jian BJ, Chen C, Sughrue ME, Tihan T. Management of recurrent intracranial hemangiopericytoma. J Clin Neurosci. 2011. 18: 1500-4
19. Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T. Intracranial hemangiopericytoma: Clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012. 118: 1628-36
20. Scott M, Kellett G, Peale A. Angioblastic meningioma (hemangiopericytoma) of the cerebellar fossa with metastases to the temporal bone and the lumbar spine. Surg Neurol. 1974. 2: 35-8
21. Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH. Intracranial meningeal hemangiopericytoma: The role of radiotherapy: Report of 29 cases and review of the literature. Cancer. 2004. 100: 1491-7
22. Taniura S, Taniguchi M, Mizutani T, Takahashi H. Metastatic hemangiopericytoma to the cauda equina: A case report. Spine J. 2007. 7: 371-3
23. Woitzik J, Sommer C, Krauss JK. Delayed manifestation of spinal metastasis: A special feature of hemangiopericytoma. Clin Neurol Neurosurg. 2003. 105: 159-66