- Neurosurgery Division, State University of Campinas, Unicamp, Sírio-Libranês, Brazil
- Neurosurgery Division, Sírio-Libranês Hospital, Sírio-Libranês, Brazil
- Neurosurgery Division, Santa Paula Hospital, Santa Paula, USA
- Department of Anesthesiology, Sírio-Libranês Hospital, Sírio-Libranês, Brazil
- Department of Radiology, Sírio-Libranês Hospital, Sírio-Libranês, Brazil
Correspondence Address:
Roger Neves Mathias
Neurosurgery Division, State University of Campinas, Unicamp, Sírio-Libranês, Brazil
Neurosurgery Division, Sírio-Libranês Hospital, Sírio-Libranês, Brazil
Neurosurgery Division, Santa Paula Hospital, Santa Paula, USA
DOI:10.4103/2152-7806.195587
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Roger Neves Mathias, Paulo Henrique Pires de Aguiar, Evandro Pinto da Luz Oliveira, Silvia Mazzali Verst, Vinícius Vieira, Marcos Fernando Docema, Marcos Vinícius Calfat Maldaun. “Next Door” intraoperative magnetic resonance imaging for awake craniotomy: Preliminary experience and technical note. 12-Dec-2016;7:
How to cite this URL: Roger Neves Mathias, Paulo Henrique Pires de Aguiar, Evandro Pinto da Luz Oliveira, Silvia Mazzali Verst, Vinícius Vieira, Marcos Fernando Docema, Marcos Vinícius Calfat Maldaun. “Next Door” intraoperative magnetic resonance imaging for awake craniotomy: Preliminary experience and technical note. 12-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/next-door-intraoperative-magnetic-resonance-imaging-for-awake-craniotomy-preliminary-experience-and-technical-note/
Abstract
Background:During glioma surgery “maximal safe resection” must be the main goal. Intraoperative magnetic resonance imaging (iMRI) associated with awake craniotomy (AC) is a valuable tool to achieve this objective. In this article, AC with a “next-door” iMRI concept is described in a stepwise protocol.
Methods:This is a retrospective analysis of 18 patients submitted to AC using iMRI; a stepwise protocol is also discussed.
Results:The mean age was 41.7 years. Hemiparesis, aphasia, and seizures were the main initial symptoms of the patients. Sixty-six percent of the tumors were located in the left hemisphere. All tumors were near or within eloquent areas. Fifty-three percent of the cases were glioblastomas multiforme and 47% of the patients had low grade gliomas. The mean surgical time and iMRI time were 4 h 4 min and 30 min, respectively. New resection was performed in 33% after iMRI. Extent of resection (EOR) higher than 95% was possible in 66.7% of the patients. The main reason of EOR lower than 95% was positive mapping of eloquent areas (6 patients). Eighty percent of the patients experienced improvement of their deficits immediately after the surgery or had a stable clinical status whereas 20% had neurological deterioration, however, all of them improved after 30 days.
Conclusion:AC associated with “next-door” iMRI is a complex procedure, but if performed using a meticulous technique, it may improve the overall tumor resection and safety of the patients.
Keywords: Awake craniotomy, glioblastoma multiforme, intraoperative magnetic resonance imaging, low grade gliomas
BACKGROUND
During glioma surgery “maximal safe resection” should always be the main concern. When brain tumors are located near or within eloquent areas, safe surgical resection can be challenging. Employing awake craniotomy (AC) techniques with cortical and subcortical mapping can define the relationship between the tumor borders and the functional areas nearby, which is crucial for a good surgical result.[
High-field (1.5 T or higher) intraoperative magnetic resonance imaging (iMRI) can improve the extent of resection (EOR).[
An iMRI set can be placed inside the operative room or can be one door away from the operating room. It can even be transoperative which means that the MRI set is located in another facility and the patient should be transported to perform the exam and return to the operation room right after. Several studies show that maximizing the EOR increases the progression-free and overall survival in both high and low-grade gliomas.[
When combining these modalities in the same procedure, concerns regarding positioning, surgical room setup, anesthesia, patient's comfort, and tolerability are critical.[
This article aims to describe the technical nuances of AC associated with “next door” iMRI surgical room. The article also discusses a clinical series of 18 cases using this methodology.
PATIENT AND METHODS
“Next door” intraoperative magnetic resonance imaging concept
An independent MRI facility with a Phillips Ingenia 1.5T MRI machine prepared to receive the surgical table was assembled one door away from the operating room. This MRI system can work independently from the surgical room maximizing cost effectiveness.
Patients and methods
Data from 18 patients diagnosed with low or high-grade gliomas infiltrating eloquent brain areas were reviewed [
Prior to the surgery, all patients were informed regarding the steps of the procedure. The tolerability and psychological profile of the patients were carefully analyzed to guarantee the safety of the procedure.
Anesthetic technique, positioning, and craniotomy
AC procedures are usually performed under an asleep–awake–asleep (AAA) technique or under sedation. We have been using a slight modification of the AAA technique described by Huncke et al.,[
All craniotomy was planned using a neuronavigation system for a tailored craniotomy. During the phase when the patient is sleeping, anesthesia is maintained with propofol and remifentanil (0.05–0.2 μg/kg/min). It is important to use a drug that has a fast metabolism and does not affect electrophysiological monitoring. Ropivacaine 0.5% with epinephrine is also used along the skin incision. After the craniotomy flap is elevated, anesthetic block of the duramater is performed using a 1:1 mixture of 1% lidocaine and 0.25% bupivacaine. Once the dura is opened, the patient is gradually awakened. At this point, all medications are stopped; usually within 20–30 min. The concern regarding analgesia, hemodynamic control, nausea and vomiting, and seizures guide the anesthesiologist's actions during this phase. If the patient becomes uncomfortable during the resection, remifentanil (0.02 μg/kg/min) is restarted. Under sedation, ventilation failure, CO2 retention, and brain swelling are also a concern.
After tumor resection, we no longer induce new anesthesia. The patient is kept awake during the rest of the procedure (iMRI and closure). All the principles of the “awake” phase are followed in this step. An easy and accessible route to the patient is essential in case the anesthesiologist needs to protect the patient's airway with an LM [
Electrophysiological monitoring and surgical technique
When indicated, cortical grid and monopolar stimulation are used to identify the motor and sensitive cortex [
When language areas are identified, the resection margin stays 1–2 cm away from these cortical areas. Regarding primary motor and sensory regions, the resection margin is taken up to 0.5 cm away. The resection is stopped if speech function deteriorates but restarts if full recovery occurs within 5 min. Cold saline solution is used to abort any seizure during neurostimulation or tumor removal. Once the resection is completed as safely as possible, the patient is prepared for the “next-door” iMRI.
Intraoperative magnetic resonance imaging technique, patient transport, and new resection
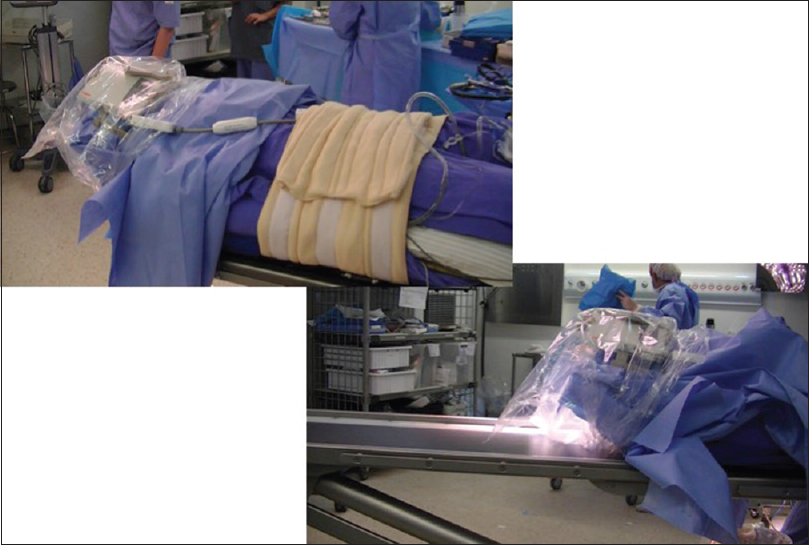
After initial resection and meticulous hemostasis is obtained, the surgical bed is filled with wet gelfoam to secure the edges in place and surgical cavity open. No hemostatic agent is used which can be confused with residual tumor. After that, the dura and skin incision is partially approximated. The wound is sterile draped, the Noras-MRI head system is assembled, and the head is sealed in a sterile bag. All electrophysiological monitoring (EPM) needles are removed from the patient. Supplementary oxygen is offered to the patient, which is kept under light sedation with dexmethomedine or propofol. The “next-door” MRI is kept waiting and prepared for the patient after proper cleaning of the facility. A rolling table is used to move the patient from the operating table to the transporting stretcher and then to the MRI table [
All devices not allowed to enter in the MRI room should be removed from the patient and a trained nurse completes a check-list during this step. The iMRI sequences for gliomas typically consist of axial images with T1 weighting obtained both pre and post-administration of gadolinium and T2 weighting, as well as any other sequences or planes of section that the surgeon and neuroradiologist deem appropriate. The most pertinent sequences such as a T1 postcontrast sequence for an enhancing malignant glioma or a T2/flair sequences for nonenhancing low-grade gliomas are acquired as 1-mm nonoverlapping contiguous slices. Consultation with the neuroradiologists is important to review the findings of the iMRI.
If any residual tumor is identified on the iMRI and is amenable for resection (not in proximity to eloquent cortical or subcortical areas), preparation for further resection is done. Important iMRI sequences are reloaded into the software of the frameless stereotactic surgical navigation system and co-registered with the preoperative surgical navigation MRIs that were used before the start of the surgery. The route back to operative room is done as well as the removal of Noras-MRI head system. The drapes that were placed before the iMRI are removed and new ones are used. Scalp retraction is re-established. Using the surgical navigation, the suspicious areas are inspected and further resection is performed. If the surgeon thinks that additional iMRI visualization would be informative, a second iMRI could be obtained; however, typically, we have not found this necessary because the areas of residual tumor are small and well localized. When possible, any additional specimens removed after the iMRI are sent as separate pathological specimens so that we can identify the pathological nature of any additional tissue removed. When the surgeon feels that the resection is completed, the craniotomy is closed in the usual fashion.
RESULTS
Patients and tumor characteristics
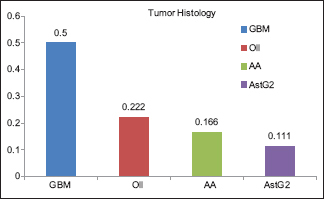
The mean age of the patients submitted to this type of surgery was 42.5 years, ranging from 29 to 57 years. Sixty-six percent of the patients were males and 33.3% females. All cases presented with neurological symptoms such as seizure, hemiparesis, aphasia, and headache. Graph 1 shows the distribution between the different tumor types encountered in this case series.
Relationship between anatomical location and eloquent areas
Seventy-two percent of the patients had tumors located in the left hemisphere; none was bifrontal. Sixty-seven percent of the tumors were located in the frontal lobe or frontoinsular portion of the brain, and 33.3% in the temporal lobe or tempoparietal portion of the brain. In all patients, eloquent areas were infiltrated by the tumor, and in all patients more than one eloquent region was affected such as Broca area, Wernick area, motor area, superior longitudinal fascicle, inferior fronto-occipital fascicle, uncinate fascicle, and corticospinal tract [
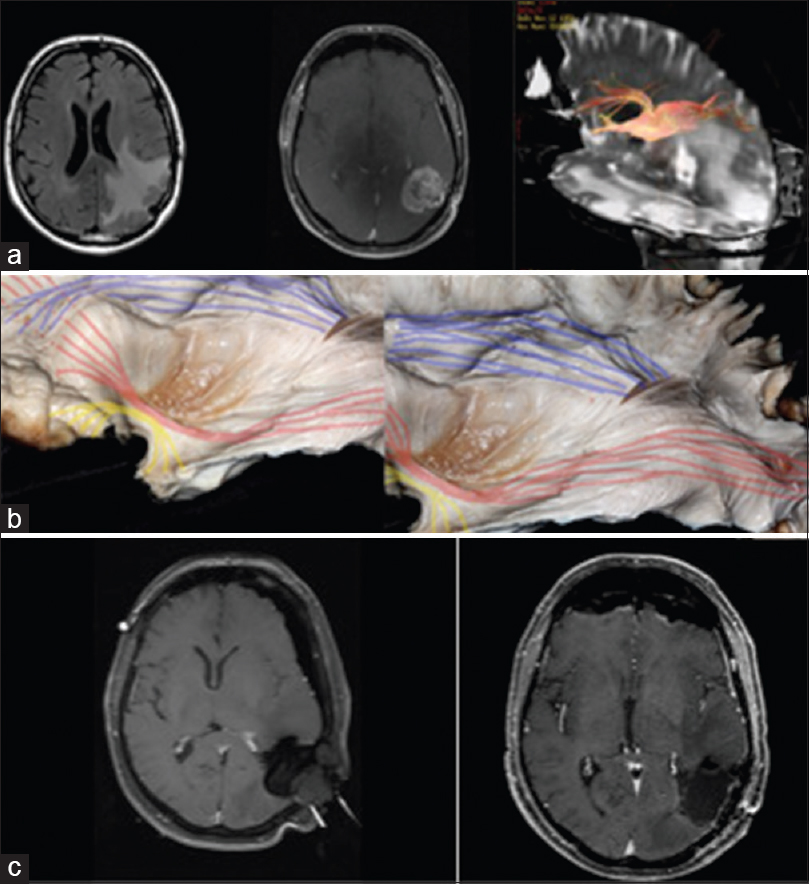
Figure 4
(a) Case of parietal GBM with involvement of the posterior portions of the superior longitudinal fascicle and superior part of the inferior frontal-occipital fascicle. DTI showing tumor involvement of the SLF. (b) cadaveric white matter dissection of the AF (blue) and IFOF (re). (c) iMRI and postoperative MRI showing GTR
Surgical time and intraoperative magnetic resonance imaging time
The mean surgical time was 4.72 ± 0.75 hours, ranging from 4 to 6 hours. The iMRI time was 30.5 ± 9.2 min, ranging from 20 to 50 min depending on the number of series acquired.
New resection after intraoperative magnetic resonance imaging and extent of resection higher than 95%
In 38.9% of the patients, new resections were needed after iMRI because residual tumor was noticed and considered removable. All patients were kept awake during this phase of the surgery. Resection rate higher than 95% of the initial tumor was achieved in 66.7% of the cases.
Intraoperative magnetic resonance imaging and the extent of resection
In the group that achieved more than 95% resection rate, the iMRI was found to be helpful in 33.3% of the cases.
Resections lower than 95%
In all 6 patients in whom the resection rate was lower than 95%, the reason was positive cortical-subcortical functional stimulation, speech arrest, or transitory muscular weakness.
Infections and stay in the hospital
The mean duration that patients stayed in the hospital was for 4.1 days, and no infections were noticed (even during the follow up).
Neurological status
In this series, 33.3% of the cases experienced neurological improvement after the surgery and 46.7% remained with their neurological status unchanged. Twenty percent of the patients had a worsening of the immediate neurological status, however, all of them returned to their basal clinical status after 30 postoperative days.
DISCUSSION
In this article, we discuss our series of 18 patients who underwent glioma resection in eloquent areas using the sleep–awake technique combined with multimodal electrophysiological monitoring and “next door” iMRI.
Using the principles of minimally invasive surgery, with tailored craniotomy and tumor resection respecting pial planes, the surgical time (mean: 4.76 hours) and blood loss are diminished. This permits the patient to be kept awake during two-thirds of the procedure, including the iMRI exam that usually is performed with the patient under light sedation.
Normally, we try to perform limited radiological study, with four main sequences in the majority of the cases (T1, T2, flair, and T1 post contrast). The 1.5-T iMRI provides good imaging quality and is a useful tool during the resection of tumors within eloquent brain areas, however, the use of MRI with such strong field in the operating facility can be danger because of the permanent magnetic attraction, and precautions must be taken to prevent projectile effect.[
From our viewpoint, a surgery that follows a stepwise protocol is important in the postoperative period. All patients went fully awake in the intensive therapy unit where normally patients stay for one night, receivinh hospital discharge in approximately 4.1 days. In addition, no infection was noticed during the follow-up of these patients. In one case after a seizure with brain swelling, the patient required longer rehabilitation during the hospital stay (14 days), fully recovering during this period.
Preoperatively, all cases presented with symptomatic gliomas in eloquent areas. It was noticed that 38.9% improved immediately after surgery. Patients who experienced postoperative worsening had neurological recovery to their basal status within 30 days. In this study, iMRI was shown to be important in the amount of tumor resection in 33.3% of the cases because resectable residual tumor was noticed in the exam. In all cases, the patients remained awake providing continuous neurological monitoring. In cases where gross total resection was not possible (6 cases), the main causes were positive stimulation and speech arrest. Even if the iMRI showed residual tumor, it was not resectable. Transport to the “next door” iMRI was quick and did not add morbidity risk to the patients.
Although transport to a nonsterile room can be a critical point, adequate sterile draping of the wound and the patient proved to be safe because no patient showed signs of infection, even during the follow up.
The combination of AC and iMRI improved the chances of obtaining “maximal safe resection.” This technique proved to be safe and useful even for high-grade gliomas, which used to be associated with worse results during AC, allowing the removal of enhanced areas in a safe manner. Some authors do not suggest AC for high grade gliomas, however, in our view, the maximal safe resection concept can also be applied for this group of patient. The combination of AC and iMRI in this small group of patients who were properly selected had similar results regarding effectiveness and safety compared with low grade gliomas.
Future studies comparing AC or iMRI used in isolation with this combined technique should be done.
CONCLUSION
The goal of glioma surgery is maximal safe resection. Combining the techniques of AC and “next door” iMRI in a step-by-step protocol proved to be a useful tool to achieve aggressiveness in glioma resection in a safe manner, preserving the quality of life.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994. 74: 1784-91
2. Berger MS, Kincaid J, Ojemann GA, Lettich E. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors. Neurosurgery. 1989. 25: 786-92
3. Bergsneider M, Sehati N, Villablanca P, McArthur DL, Becker DP, Liau LM. Mahaley Clinical Research Award: Extent of glioma resection using low-field (0.2 T) versus high-field (1.5 T) Intraoperative MRI and image-guided frameless neuronavigation. Clin Neurosurg. 2005. 52: 389-99
4. Black PM, Alexander E, Martin C, Moriarty T, Nabavi A, Wong T. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery. 1999. 45: 423-31
5. Black PM, Moriarty T, Alexander E, Stieg P, Woodard EJ, Gleason PL. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997. 41: 831-42
6. Bohinski RJ, Warnick RE, Gaskill-Shipley MF, Zucarello M, van Loveren HR, Kormos DW. Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macro-adenomas during transsphenoidal microsurgery. Neurosurgery. 2001. 49: 1133-43
7. Claus EB, Horlacher A, Hsu L, Schawartz RB, Dello-lacono D, Talos F. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005. 103: 1227-33
8. Fahlbusch R, Keller B, Ganslandt O, Kreutzer J, Nimsky C. Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur J Endocrinol. 2005. 153: 239-48
9. Huncke K, Van de Wiele B, Fried I, Rubinstein EH. The asleep-awake-asleep anesthetic technique for intraoperative language mapping. Neurosurgery. 1998. 42: 1312-7
10. Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Usgard G. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012. 308: 1881-8
11. Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: A critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001. 95: 735-45
12. Kim SS, McCutcheon IE, Suki D, Weinberg JS, Sawaya R, Lang FF. Awake craniotomy for brain tumors near eloquent cortex: Correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery. 2009. 64: 836-46
13. Knauth M, Wirtz CR, Tronnier VM, Aras N, Kunze S, Sartor K. Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am J Neuroradiol. 1999. 20: 1642-6
14. Kremer P, Tronnier V, Steiner HH, Edinger F, Rating D, Hartmann M. Intraoperative MRI for interventional neurosurgical procedures and tumor resection control in children. Childs Nerv Syst. 2006. 22: 674-8
15. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, Demonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
16. Leuthardt EC, Lim CC, Shah MN, Evans JA, Rich KM, Dacey RG. Use of movable high-field-strength intraoperative magnetic resonance imaging with awake craniotomies for resection of gliomas: Preliminary experience. Neurosurgery. 2011. 69: 194-205
17. Maldaun MV, Khawja SN, Levine NB, Rao G, Lang FF, Weinberg JS. Awake craniotomy for gliomas in a high-field intraoperative magnetic resonance imaging suite: Analysis of 42 cases. J Neurosurg. 2014. 8: 1-8
18. Martin AJ, Hall WA, Liu H, Pozza CH, Michel E, Casey SO. Brain tumor resection: Intraoperative monitoring with high- field-strength MR imaging-Initial results. Radiology. 2000. 215: 221-8
19. Martin CH, Schwartz R, Jolesz F, Black PM. Transsphenoidal resection of pituitary adenomas in an intraoperative MRI unit. Pituitary. 1999. 2: 155-62
20. Nimsky C, Ganslandt O, Von Keller B, Romstock J, Fahlbusch R. Intraoperative high-field-strength MR imaging: Implementation and experience in 200 patients. Radiology. 2004. 233: 67-78
21. Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere: An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989. 71: 316-26
22. Parney IF, Goerss SJ, McGee K, Huston J, Perkins WJ, Meyer FB. Awake craniotomy, electrophysiologic mapping, and tumor resection with high-field intraoperative MRI. World Neurosurg. 2010. 73: 547-51
23. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008. 358: 18-27