- Departments of Neurosurgery Salford Royal NHS Foundation Trust, Salford, Manchester, United Kingdom.
- Departments of Stroke Medicine, Salford Royal NHS Foundation Trust, Salford, Manchester, United Kingdom.
Correspondence Address:
Md Tanvir Hasan
Departments of Stroke Medicine, Salford Royal NHS Foundation Trust, Salford, Manchester, United Kingdom.
DOI:10.25259/SNI_481_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Md Tanvir Hasan1, Daniel Lewis1, Mohammed Siddiqui2. Brain abscess – A rare complication of endovascular treatment for acute ischemic stroke. 02-Oct-2020;11:319
How to cite this URL: Md Tanvir Hasan1, Daniel Lewis1, Mohammed Siddiqui2. Brain abscess – A rare complication of endovascular treatment for acute ischemic stroke. 02-Oct-2020;11:319. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10303
Abstract
Background: Brain abscess is a neurosurgical emergency, which can arise through direct bacterial seeding or hematogenous spread. Rarely, brain abscess formation has been reported following ischemic stroke. An increasingly utilized therapy for stroke is mechanical thrombectomy, and within this report, we present a case of brain abscess formation following this procedure.
Case Description: A 78-year-old female presented to our center with a right total anterior circulation stroke (TACS) secondary to terminal internal carotid artery occlusion. An emergent mechanical thrombectomy was performed and the patient’s initial postoperative recovery was good. In the 3rd week after the procedure, however, the patient became more confused and following the onset of fever, an MRI brain was performed, which demonstrated an extensive multiloculated right-sided brain abscess. Burr hole drainage of the abscess was subsequently undertaken and pus samples obtained grew Proteus mirabilis, presumed secondary to a urinary tract infection, and the patient was started on prolonged antibiotic therapy. To date, the infection has been eradicated and the patient survives albeit with persistent neurological deficits.
Conclusion: To the best of our knowledge, this is the first reported UK case of brain abscess following mechanical thrombectomy for stroke. Endovascular interventions can lead to increased incidence of ischemia-reperfusion injury in stroke with increased blood–brain barrier damage and risk of microbial seeding. This case highlights the need for rigorous asepsis and proactive treatment of systemic infections in the acute phase following endovascular treatment and consideration of brain abscess in all patients who present with new-onset confusion and unexplained fever following stroke.
Keywords: Brain Abscess, Stroke, Thrombectomy
INTRODUCTION
Brain abscess is a rare but potentially life-threatening condition, which is caused by bacterial seeding into the brain parenchyma.[
CASE DESCRIPTION
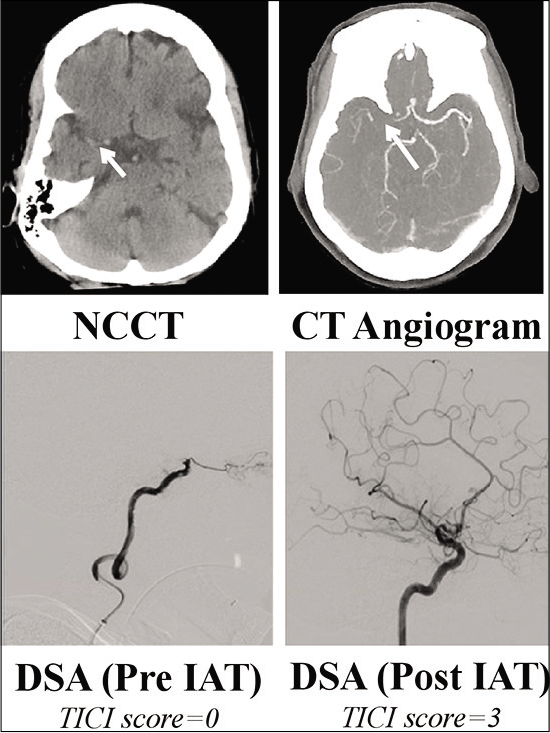
A 78-year-old previously fit and independent lady presented to our center 3½ hours after witnessed onset of left-sided weakness and difficulty in speaking following a fall. Relevant medical history included atrial fibrillation for which she was taking apixaban and mild cognitive impairment; premorbid modified Rankin scale was 1. On admission, the patient was seen by the specialist stroke service and diagnosed with a right total anterior circulation stroke (TACS); NIHSS score was 21. Noncontrast CT head and intracranial CT angiography demonstrated an extensive right MCA territory infarction with occlusion of the right terminal internal carotid artery (ICA) and right proximal M1 segment middle cerebral artery (MCA) [
Following the thrombectomy procedure, the patient initially made a good postoperative recovery, recovering to Medical Research Council Grade 3 in arm and leg, and the patient was repatriated back to her local hospital. In the 3rd week after repatriation, however, the patient developed progressive confusion. Repeat CT head at 4 weeks postthrombectomy was initially reported as demonstrating possible hemorrhagic transformation with surrounding edema and midline shift.
Figure 1:
Top: admission noncontrast CT (NCCT) head demonstrated extensive right MCA territory infarction with hyperdense right MCA M1 segment (short arrow). ASPECT score was 6. CT angiogram confirmed occlusion of the terminal right ICA and right M1 segment (long arrow). Note retrograde flow within the A1 vessel from the contralateral side. Bottom: catheter angiography images (arterial phase) pre and post intra-arterial thrombectomy showing successful recanalization of the terminal ICA and right MCA. TICI score pre- and postintervention was 0 and 3, respectively. ASPECT: Alberta stroke program early CT score; DSA: Digital subtraction angiography, ICA: Internal carotid artery, MCA: Middle cerebral artery, NCCT: Noncontrast CT, TICI: Thrombolysis in cerebral infarction.
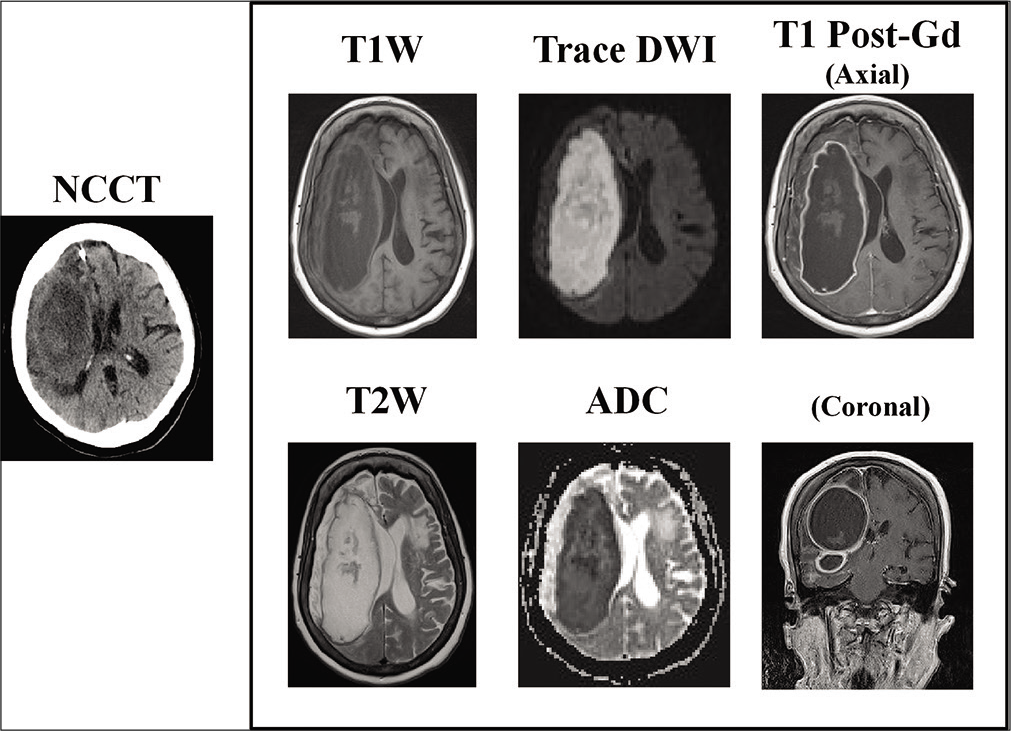
Due to ongoing confusion and the development of pyrexia, further imaging with both contrast-enhanced CT and MRI was undertaken at 7 weeks posttreatment. MR imaging demonstrated an extensive right frontal cerebral abscess with mass effect and midline shift [
Figure 2:
Noncontrast computed tomography (NCCT) and contrast-enhanced MR imaging performed 7 weeks postthrombectomy. T1- and T2-weighted images demonstrate am extensive right-sided multiloculated fluid-filled cavity extending into the right middle cranial fossa, right temporal pole, and right inferior frontal lobe. Trace DWI/ADC images demonstrate prominent diffusion restriction consistent with abscess formation and on post-Gd T1W images there is marked enhancement of the abscess capsule peripherally. Note the high T2-weighted signal change within the surrounding right frontoparietal lobes, and the associated mass effect from the abscess with partial effacement of the right lateral ventricle and midline shift. ADC: Apparent diffusion coefficient; DWI: Diffusion-weighted imaging, T1W: T1 weighted, T2W: T2 weighted.
DISCUSSION
Mechanical thrombectomy has an established and growing role in the management of ischemic stroke and similar to other endovascular techniques is generally considered to be a clean procedure.[
In two of the three previously reported cases of abscess formation following endovascular treatment [
The classic presentation of brain abscess is with fever, headache, and focal neurological deficit but in only 20% of cases is this classic triad of symptoms seen.[
The above case and the included literature review highlight that brain abscess should be considered in the differential diagnosis in any poststroke patient who presents with severe headache, fever, or new-onset confusion, irrespective of whether any endovascular or surgical intervention has been undertaken.[
CONCLUSION
To the best of our knowledge, this is one of only four reported cases of brain abscess following endovascular therapy for stroke and the first such reported case in the UK. Our case highlights the need for rigorous asepsis and proactive treatment of systemic infections in the periprocedural and acute phase following endovascular stroke therapy and the requirement to consider this rare, but potentially life-threatening complication in all patients who present with unexplained fever, severe headache, or new-onset confusion following stroke.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akuzawa N, Osawa T, Totsuka M, Hatori T, Imai K, Kitahara Y. Secondary brain abscess following simple renal cyst infection: A case report. BMC Neurol. 2014. 14: 130
2. AL-Okaili R, Patel SJ. Brain abscess after endovascular coiling of a saccular aneurysm: Case report. AJNR Am J Neuroradiol. 2002. 23: 697-9
3. Beloosesky Y, Streifler JY, Eynan N, Grinblat J. Brain abscess complicating cerebral infarct. Age Ageing. 2002. 31: 477-80
4. Bodilsen J, Dalager-Pedersen M, van de Beek D, Brouwer MC, Nielsen H. Risk factors for brain abscess: A nationwide, population-based, nested case-control study. Clin Infect Dis. 2020. 71: 1040-6
5. Boukobza M, Nahmani S, Deschamps L, Laissy JP. Brain abscess complicating ischemic embolic stroke in a patient with cardiac papillary fibroelastoma-case report and literature review. J Clin Neurosci. 2019. 66: 277-9
6. Chen ST, Tang LM, Ro LS. Brain abscess as a complication of stroke. Stroke. 1995. 26: 696-8
7. Davis MC, Deveikis JP, Harrigan MR. Clinical presentation, imaging, and management of complications due to neurointerventional procedures. Semin Intervent Radiol. 2015. 32: 98-107
8. Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T. Stroke-induced immunodepression: Experimental evidence and clinical relevance, in stroke. Stroke. 2007. 38: 770-3
9. Goodkin HP, Harper MB, Pomeroy SL. Intracerebral abscess in children: Historical trends at children’s hospital Boston. Pediatrics. 2004. 113: 1765-70
10. Guenego A, Rafiq M, Michelozzi C, Januel AC, Albucher JF, Sol JC. Secondary cerebral abscess of an ischemic stroke treated by thrombectomy. J Neuroradiol. 2017. 44: 403-6
11. Hug A, Dalpke A, Wieczorek N, Giese T, Lorenz A, Auffarth G. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009. 40: 3226-32
12. Inamasu J, Kagami H, Nakamura Y, Saito R, Niimi M, Ichikizaki K. Brain abscess developing at the site of preceding intracerebral hemorrhage. J Neurol. 2002. 249: 221-3
13. Jabre R, Bernat AL, Peres R, Froelich S. Brain abscesses after endovascular embolization of a brain arteriovenous malformation with squid. World Neurosurg. 2019. 132: 29-32
14. Kelkar PS, Fleming JB, Walters BC, Harrigan MR. Infection risk in neurointervention and cerebral angiography. Neurosurgery. 2013. 72: 327-31
15. Ko JH, Kim YJ, Jung HH. Brain abscess after stent-assisted coiling for ruptured middle cerebral artery aneurysm. Interv Neuroradiol. 2018. 24: 387-91
16. Kraemer JL, Worm PV, Faria MD, Maulaz A. Brain abscess following ischemic stroke with secondary hemorrhage. Arq Neuropsiquiatr. 2008. 66: 104-6
17. Lange N, Berndt M, Jörger AK, Wagner A, Lummel N, Ryang YM. Clinical characteristics and course of postoperative brain abscess. World Neurosurg. 2018. 120: e675-83
18. Mada PK, Morris L, Katikaneni P, Chandranesan AS. Angio-seal infection: An uncommon but serious and alarming complication of vascular closure devices. Vasc Dis Ther. 2017. 2: 1-2
19. Meyer P, Reizine D, Aymard A, Guerin JM, Merland JJ, Habib Y. Septic complications in interventional angiography: Evaluation of risk and preventive measures. Preliminary studies. J Interv Radiol. 1988. 3: 73-5
20. Miyazaki H, Ito S, Nitta Y, Iino N, Shiokawa Y, de Bruijn SF. Brain abscess following cerebral infarction. Acta Neurochir (Wien). 2004. 146: 531-2
21. Niego B, Medcalf RL. Plasmin-dependent modulation of the blood-brain barrier: A major consideration during tPA-induced thrombolysis?. J Cereb Blood Flow Metab. 2014. 34: 1283-96
22. Rao SK, Ahmad O, Tariq F, Suchdev K, Mittal S, Mohamed W. Cerebral abscess following mechanical thrombectomy for ischemic stroke: Report of a case and review of literature. Cureus. 2018. 10: e2824
23. Reddy JS, Mishra AM, Behari S, Husain M, Gupta V, Rastogi M. The role of diffusion-weighted imaging in the differential diagnosis of intracranial cystic mass lesions: A report of 147 lesions. Surg Neurol. 2006. 66: 246-50
24. Smilowitz NR, Kirtane AJ, Guiry M, Gray WA, Dolcimascolo P, Querijero M. Practices and complications of vascular closure devices and manual compression in patients undergoing elective transfemoral coronary procedures. Am J Cardiol. 2012. 110: 177-82
25. Stienen MN, Moser N, Krauss P, Regli L, Sarnthein J. Incidence, depth, and severity of surgical site infections after neurosurgical interventions. Acta Neurochir (Wien). 2019. 161: 17-24
26. Wang J, Fraser JF. An intracranial petri dish? formation of abscess in prior large stroke after decompressive hemicraniectomy. World Neurosurg. 2015. 84: 1495.e5-9
27. Yamanaka K, Ishihara M, Nakajima S, Yamasaki M, Yoshimine T. Brain abscess following intra-arterial thrombolytic treatment for acute brain ischemia. J Clin Neurosci. 2011. 18: 968-70
28. Zurin AAR, Ushikoshi S, Houkin K, Kikuchi Y, Abe H, Saitoh H. Cerebral abscess as an unusual complication of coil embolization in a dural arteriovenous fistula. Case report. J Neurosurg. 1997. 87: 109-12