- Department of Neurosurgery, Sapporo Teishinkai Hospital, Higashi-ku, Sapporo, Japan.
DOI:10.25259/SNI_99_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nakao Ota, Johan Carlos Valenzuela, Daiki Chida, Rokuya Tanikawa. Extracranial vertebral artery to middle cerebral artery bypass in therapeutic internal carotid artery occlusion for epipharyngeal carcinoma: A technical case report. 08-Apr-2021;12:149
How to cite this URL: Nakao Ota, Johan Carlos Valenzuela, Daiki Chida, Rokuya Tanikawa. Extracranial vertebral artery to middle cerebral artery bypass in therapeutic internal carotid artery occlusion for epipharyngeal carcinoma: A technical case report. 08-Apr-2021;12:149. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10694
Abstract
Background: Vertebral artery (VA) to middle cerebral artery (MCA) bypass is a rarely selected technique because a complex expanded dissection is required, and often, a better donor artery than VA exists. A good indication for VA-MCA bypass is the treatment of head-and-neck malignancies with the sacrifice of the internal carotid artery (ICA) or for carotid artery rupture.
Methods: A 23-year-old man with epipharyngeal carcinoma, treated by ligating the carotid artery with a VAMCA bypass before chemoradiotherapy, was reported. Radiographic findings showed that the bone of the carotid canal was dissolved, and the right ICA was engulfed by the tumor. As epipharyngeal carcinoma is hypersensitive to radiation, in cases where the tumor rapidly disappears, ICA may dangle in the pharynx and rupture may occur. In addition, to irradiate sufficiently, the ICA may become an obstacle. Hence, we decided to perform carotid ligation with a VA-MCA bypass before radiation and chemotherapy for the primary lesion. We selected the V3 portion of the VA as the donor on the ipsilateral side, as it can supply high-flow cerebral blood flow, which is not influenced by carcinoma and less influenced by irradiation for the epipharynx.
Results: The VA-MCA bypass was completed without complications followed by endovascular occlusion of the ICA. Induction chemotherapy was initiated for the patient 2 weeks after surgery. The patient achieved a complete response following chemoradiotherapy.
Conclusion: ICA ligation with VA-MCA high-flow bypass earlier than chemoradiotherapy is useful for epipharyngeal carcinoma as it prevents carotid artery rupture and allows radical intervention.
Keywords: Carotid artery rupture, Carotid blowout syndrome, Epipharyngeal carcinoma, Skull base malignancy, Vertebral artery to middle cerebral artery bypass
INTRODUCTION
Vertebral artery to middle cerebral artery (VA-MCA) bypass is a rarely selected technique as exposing the extracranial VA takes time, and sometimes, other recipient arteries are more useful to reach MCA, such as an external carotid artery or superficial temporal artery (STA). However, the VA-MCA bypass technique can be a good alternative in cases where another recipient artery cannot be selected.[
CBS is a rare but severe complication after surgical and radiological interventions for head-and-neck cancers.[
To avoid ischemic complications, carotid ligation with bypass or endovascular repair with covered stents should be considered as another option.[
Here, we present a case of planned carotid ligation preceding the intervention for epipharyngeal carcinoma with VA-MCA bypass.
CASE PRESENTATION
This study was approved by the Institutional Review Board, and informed consent was obtained from the patient. A 23-year-old man presented with a 3-month history of progressive difficulty in swallowing and discomfort in the pharynx. The patient was diagnosed with epipharyngeal carcinoma using biopsy. The pathology report revealed nonkeratinizing nasopharyngeal carcinoma, undifferentiated type, Ki-labeling index 60–70%, and latent membrane protein-1 positive, cT4N0M0, cSTAGE IV A.
Computed tomography (CT), CT angiography (CTA), and magnetic resonance imaging (MRI) revealed a heterogeneous mass occupying the epipharyngeal region [
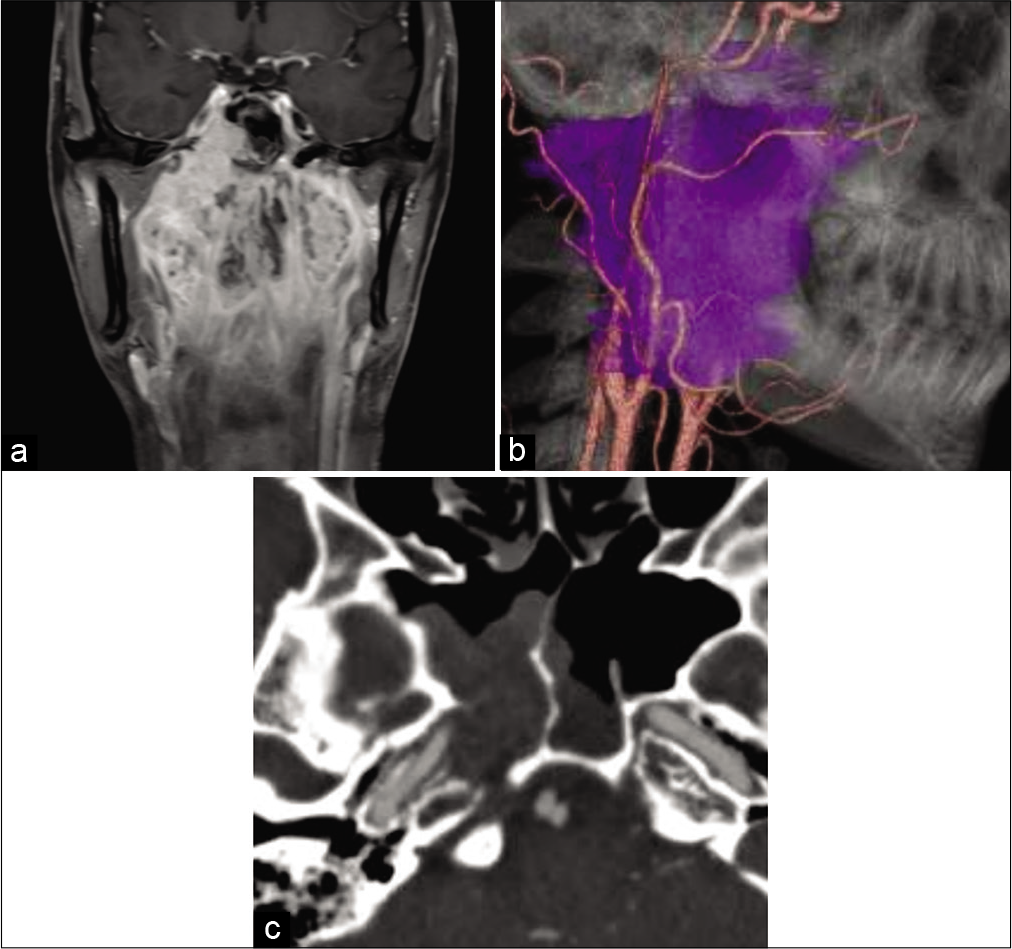
Figure 1:
Preoperative images of the case. (a) Coronal gadolinium-enhanced T1-weighted magnetic resonance imaging shows that the tumor envelops the epipharyngeal region to the sphenoid sinus and the skull base. (b) Lateral view of fusion image between computed tomography angiography and tumor (purple color) reconstructions. Tumor engulfs the right side internal and external carotid arteries. (c) Computed tomography angiography reveals that the tumor envelopes the sphenoid sinus and the right side carotid canal. The bony structure of the carotid canal is dissolved by the tumor.
Preoperative balloon test occlusion (BTO) revealed 35% decrease in ICA pressure compared with preocclusion ICA pressure. We decided to perform ICA ligation using a V3- radial artery graft (RAG)-MCA bypass in anticipation of radiation and chemotherapy for the primary lesion. We selected the V3 portion of the VA as the donor on the ipsilateral side, as it can supply high-flow cerebral blood flow not influenced by carcinoma and is less influenced by irradiation of the epipharynx.
Surgical technique
Supplementary video shows the detailed step-by-step procedures [
Video 1
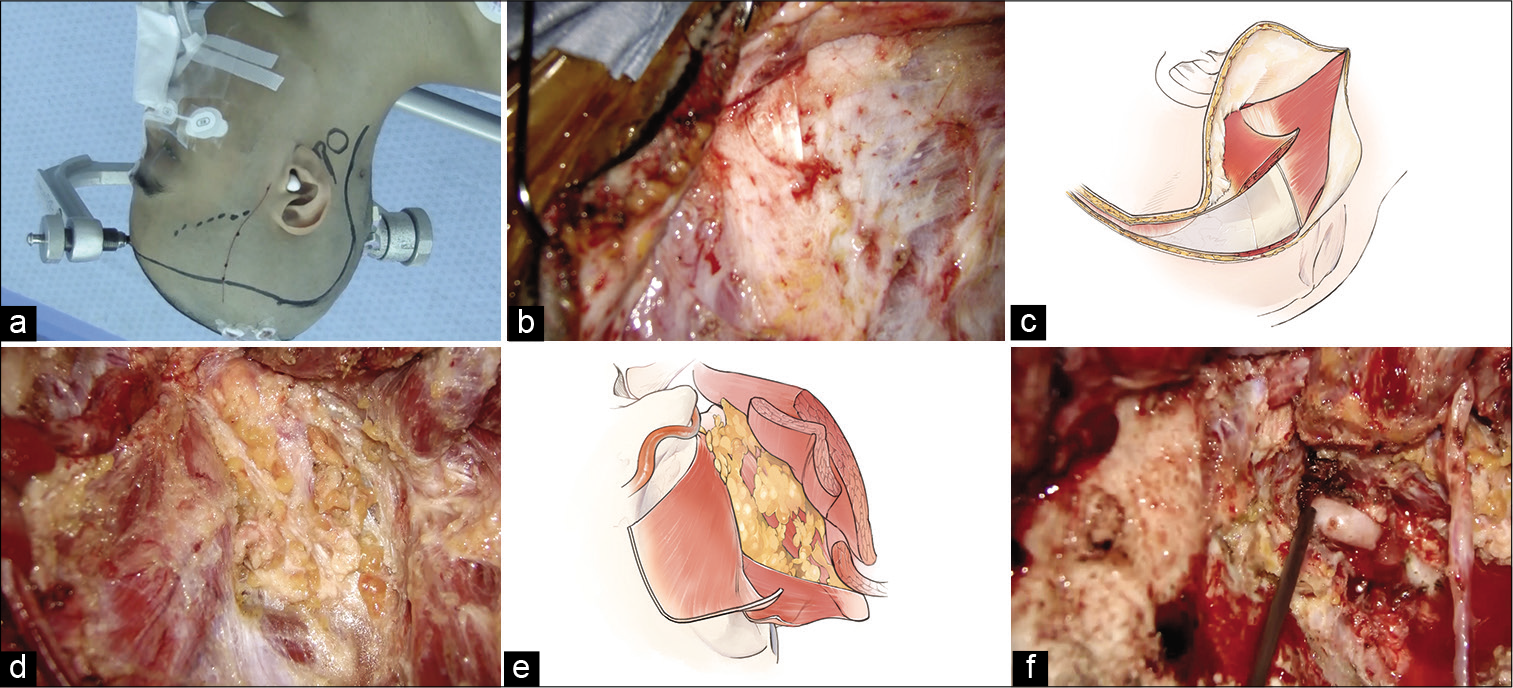
Figure 2:
Intraoperative findings. (a) Right skin incision design. The frontotemporal skin incision is designed from just above the superficial temporal artery parietal branch to the hair’s midline. To join this skin incision, an additional curved one along the lateral process of the C2 vertebra is added. (b) The sternocleidomastoid and occipital muscles are exposed. (c) The occipital muscle is elevated as it belongs to the skin flap by detaching it from the superior nuchal line. (d and e) The suboccipital triangle is exposed. (f) The V3 portion of the vertebral artery is exposed.
First, the parietal branch of the STA was harvested from just below the skin incision, and the frontotemporal skin flap was elevated in a two-layer manner. The frontal branch of the STA was harvested from the skin flap. An additional skin incision was made to join this frontotemporal skin incision. The galea and occipital muscles were dissected to belong to the skin flap. To do this, the sternocleidomastoid (SCM) muscle and occipital muscle were exposed first [
Subsequently, a frontotemporal craniotomy was performed. By distal Sylvian fissure dissection, the M1 to M3 portion of the MCA was fully exposed [
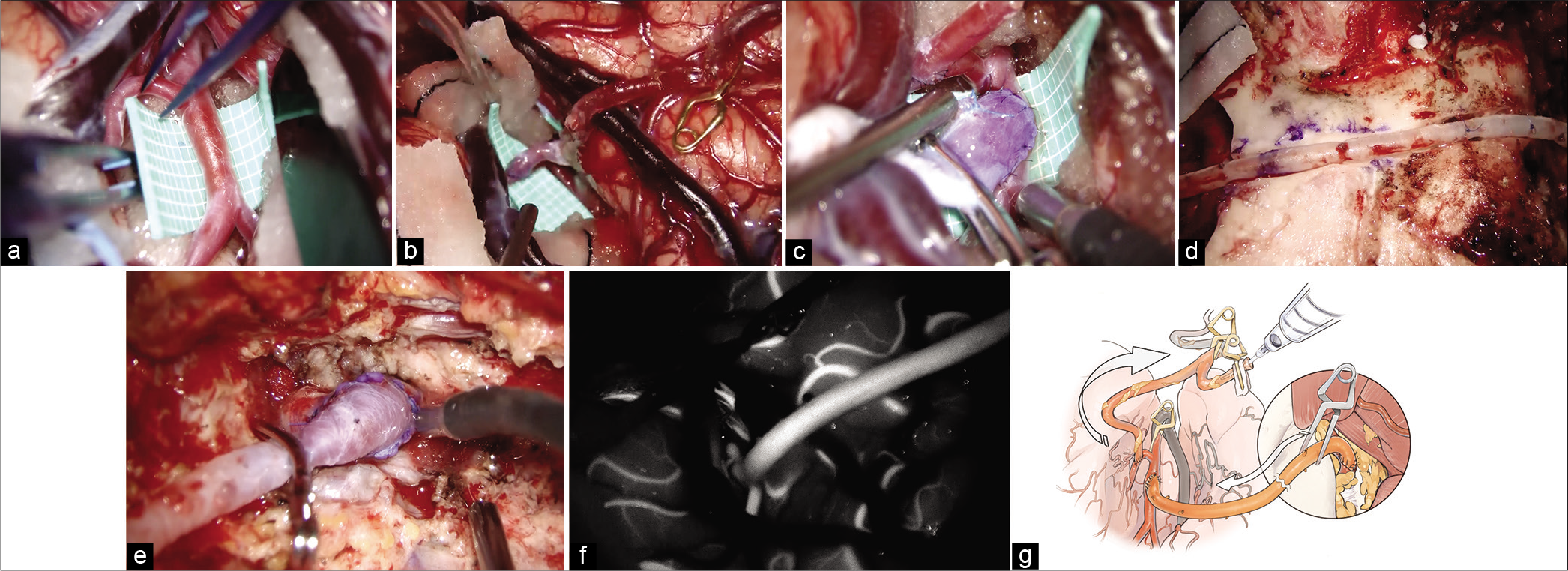
Figure 3:
Intraoperative findings of the V3-radial artery graft -M2 bypass. (a) Superior trunk of the M2 is considered as a recipient artery once Sylvian fissure dissection is complete. (b) At first, superficial temporal artery to the M3 portion of the middle cerebral artery (MCA) bypass is performed. (c) The M2 portion of the MCA to RAG anastomosis is performed. (d) The mastoid and the temporal bones are drilled just below the graft route not to compress the graft and make the graft route shorter. (e) V3 to RAG anastomosis is performed. (f) Indocyanine green video angiography demonstrates good filling of the V3-RAG-M2 bypass. (g) Schematic image of the pressure monitoring.
Postoperative course
The patient was conscious, with no neurological deficit. CTA showed good patency of the V3-RAG-MCA and STAMCA bypasses. The ICA was occluded using endovascular coiling the day after systemic heparinization [
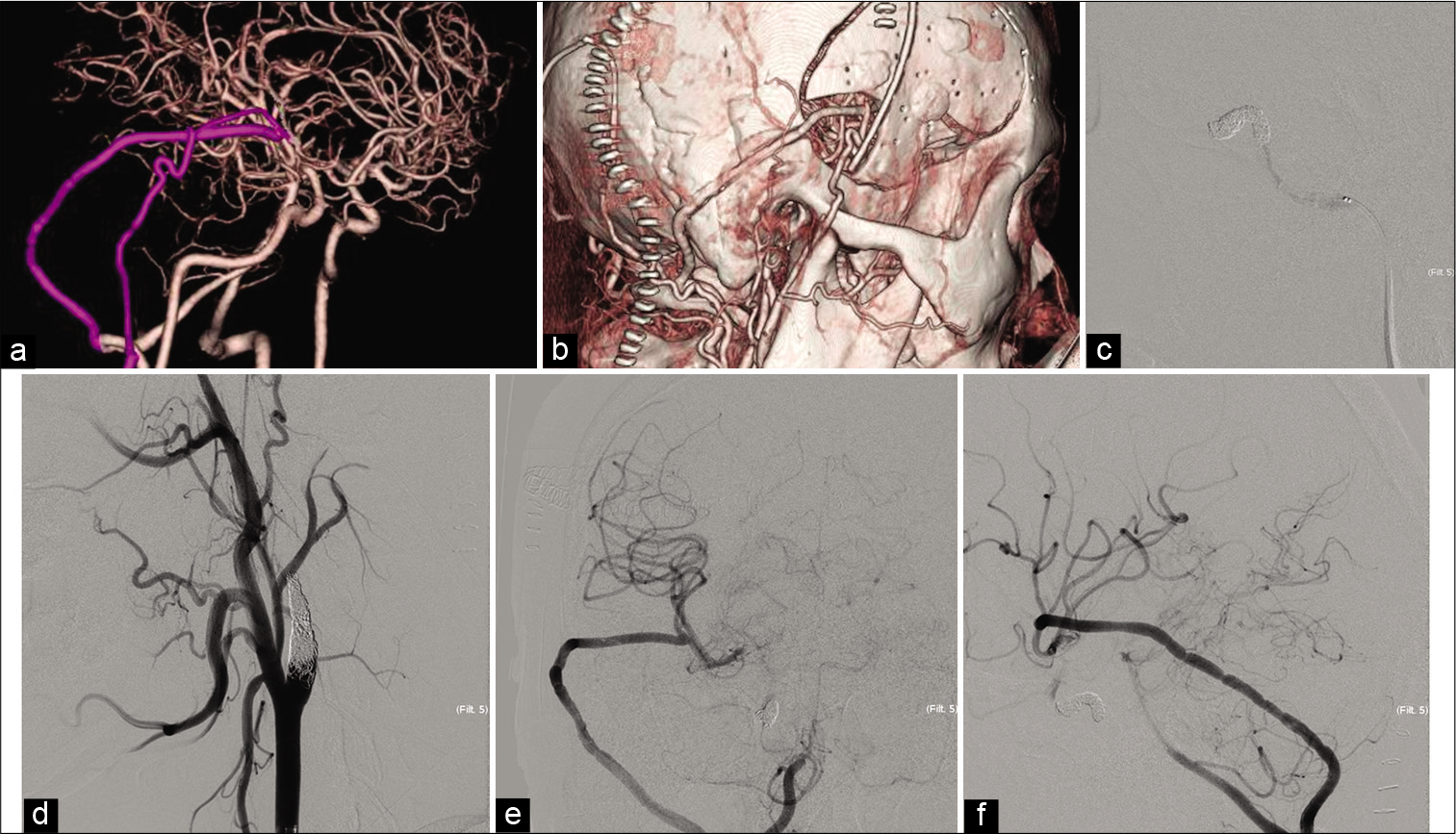
Figure 4:
Postoperative courses. (a and b) The reconstruction of the computed tomography angiography in lateral view reveals good filling of the V3-radial artery graft (RAG)-M2 and superficial temporal artery -middle cerebral artery bypass. The internal carotid artery is not occluded at this time. (c) The cavernous portion of the internal carotid artery (ICA) is occluded by coil, lateral view of angiography image. (d) The cervical ICA is occluded, oblique view of angiography image. (e and f) Anterior to the posterior and lateral view of the digital subtraction angiography after ICA occlusion shows good filling of the V3-RAG-M2 bypass.
DISCUSSION
Indication for carotid ligation with bypass before intervention for the primary lesion
Although the planned bypass for the present case was completed without complications and with the shortest time to induction chemotherapy, the indication for bypass should be carefully analyzed. Although radical neck dissection using carotid artery sacrifice with bypass for advanced skull base malignancies has been reported previously, to the best of our knowledge, this is the first report where bypass surgery was performed for epipharyngeal carcinoma. Kalani et al.[
Risk of carotid artery rupture
The incidence of CBS in major head-and-neck oncological surgeries is reported between 3% and 4.5%.[
In addition, postradiation ICA stenosis or occlusion occurs.[
Bypass indication and selection of donor artery
In patients with malignant tumors of the head and neck, the selection of the ipsilateral external carotid artery as a donor artery is challenging due to its malignant nature and as it would be irradiated before or after surgery. However, contralateral STA or ipsilateral extracranial V3 can be selected. One option is a bonnet STA-interpositional graft-MCA low-flow bypass using the contralateral STA as a donor for advanced skull base malignant tumors. Kalani et al.[
Although BTO identifies patients at risk of immediate ischemia caused by occlusion, its sensitivity is controversial.[
Limitation of this study
This is a technically successful bypass surgery case report. The prognosis of this patient and the effectiveness of the bypass are still unknown. The validity of ICA ligation with V3-MCA bypass before treating malignant tumors of the head and neck was carefully discussed by correcting more cases.
CONCLUSION
ICA ligation with VA-MCA high-flow bypass before chemoradiotherapy for epipharyngeal carcinoma is useful as it prevents carotid artery rupture and allows radical intervention. Careful indications should be considered.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
Acknowledgments
We gratefully thank Seina Inoue for her beautiful illustration.
References
1. Brinjikji W, Cloft HJ. Outcomes of endovascular occlusion and stenting in the treatment of carotid blowout. Interv Neuroradiol. 2015. 21: 543-7
2. Chen YJ, Wang CP, Wang CC, Jiang RS, Lin JC, Liu SA. Carotid blowout in patients with head and neck cancer: Associated factors and treatment outcomes. Head Neck. 2015. 37: 265-72
3. Chen YL, Wong HF, Ku YK, Wong AM, Wai YY, Ng SH. Endovascular covered stent reconstruction improved the outcomes of acute carotid blowout syndrome. Experiences at a single institute. Interv Neuroradiol. 2008. 14: 23-7
4. Cheng KY, Lee KW, Chiang FY, Ho KY, Kuo WR. Rupture of radiation-induced internal carotid artery pseudoaneurysm in a patient with nasopharyngeal carcinoma-spontaneous occlusion of carotid artery due to long-term embolizing performance. Head Neck. 2008. 30: 1132-5
5. Graffeo CS, Link MJ, Stafford SL, Parney IF, Foote RL, Pollock BE. Risk of internal carotid artery stenosis or occlusion after single-fraction radiosurgery for benign parasellar tumors. J Neurosurg. 2019. 2019: 1-8
6. Hadeishi H, Yasui N, Okamoto Y. Extracranial-intracranial high-flow bypass using the radial artery between the vertebral and middle cerebral arteries. Technical note. J Neurosurg. 1996. 85: 976-9
7. Kalani MY, Kalb S, Martirosyan NL, Lettieri SC, Spetzler RF, Porter RW. Cerebral revascularization and carotid artery resection at the skull base for treatment of advanced head and neck malignancies. J Neurosurg. 2013. 118: 637-42
8. Lu HJ, Chen KW, Chen MH, Chu PY, Tai SK, Wang LW. Predisposing factors, management, and prognostic evaluation of acute carotid blowout syndrome. J Vasc Surg. 2013. 58: 1226-35
9. Meybodi AT, Benet A, Lawton MT. The V3 segment of the vertebral artery as a robust donor for intracranialto-intracranial interpositional bypasses: Technique and application in 5 patients. J Neurosurg. 2018. 129: 691-701
10. Miele VJ, Rosen CL, Carpenter J, Rai A, Bailes JE. Vertebral artery-to-middle cerebral artery bypass with coil embolization of giant internal carotid artery aneurysm: Technical case report. Neurosurgery. 2005. 56: E1159
11. Patsalides A, Fraser JF, Smith MJ, Kraus D, Gobin YP, Riina HA. Endovascular treatment of carotid blowout syndrome: Who and how to treat. J Neurointerv Surg. 2010. 2: 87-93
12. Qiu S, Lin S, Tham IW, Pan J, Lu J, Lu JJ. Intensity-modulated radiation therapy in the salvage of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012. 83: 676-83
13. Roh JL, Suh DC, Kim MR, Lee JH, Choi JW, Choi SH. Endovascular management of carotid blowout syndrome in patients with head and neck cancers. Oral Oncol. 2008. 44: 844-50
14. Shan GP, Wang BB, Zheng P, Du FL, Yang YW. Efficacy and safety of chemotherapy combined with stereotactic radiotherapy in the treatment of nasopharyngeal carcinoma. Med Sci Monit. 2017. 23: 5630-6
15. Suarez C, Fernandez-Alvarez V, Hamoir M, Mendenhall WM, Strojan P, Quer M. Carotid blowout syndrome: Modern trends in management. Cancer Manag Res. 2018. 10: 5617-28