- Department of Neuroscience, Neurosurgery Unit, Alessandro Manzoni Hospital, Lecco, Italy.
- Department of Neurosurgery, Highly Specialized Hospital of National Importance “Garibaldi”, Catania, Italy.
- Department of Oncology, Pathological Anatomy and Histology Unit, Alessandro Manzoni Hospital, Lecco, Italy.
- Department of Surgical Specialties, Radiological Sciences and Public Health, Unit of Otorhinolaryngology – Head and neck Surgery, Piazza del Mercato, Brescia, Italy.
- Department of Neurosurgery, IRCCS Fondazione Policlinico San Matteo, University of Pavia, Pavia, Italy.
Correspondence Address:
Antonio Crea, Department of Neuroscience, Neurosurgery Unit, Alessandro Manzoni Hospital, Lecco, Italy.
DOI:10.25259/SNI_386_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Antonio Crea1, Gianluca Grimod1, Gianluca Scalia2, Mariarosaria Verlotta1, Lucio Mazzeo1, Giorgio Rossi3, Davide Mattavelli4, Vittorio Rampinelli4, Sabino Luzzi5, Giannantonio Spena1. Fronto-orbito-ethmoidal intradiploic meningiomas: A case study with systematic review. 30-Sep-2021;12:485
How to cite this URL: Antonio Crea1, Gianluca Grimod1, Gianluca Scalia2, Mariarosaria Verlotta1, Lucio Mazzeo1, Giorgio Rossi3, Davide Mattavelli4, Vittorio Rampinelli4, Sabino Luzzi5, Giannantonio Spena1. Fronto-orbito-ethmoidal intradiploic meningiomas: A case study with systematic review. 30-Sep-2021;12:485. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11142
Abstract
Background: Primary intradiploic meningiomas, extra-axial tumors arising primarily in the skull, are rare. The authors reported a complex case of intradiploic intraosseous metaplastic meningioma of the left medial wall and orbital roof with the left frontal sinus invasion and left ethmoidal body bone substitution. The authors also conducted a systematic review concerning diagnosis and management of patients affected by purely calvarial intradiploic meningiomas along with a focus on fronto-orbito-ethmoidal ones.
Methods: A literature search was conducted using PubMed and Scopus databases according to preferred reporting items for systematic reviews and meta-analysis statement and with the following Mesh terms: Intradiploic, intraosseous, calvarial, and meningioma. Eligibility criteria were limited by the nature of existing literature on intradiploic meningiomas, consisting of only case series, and case reports.
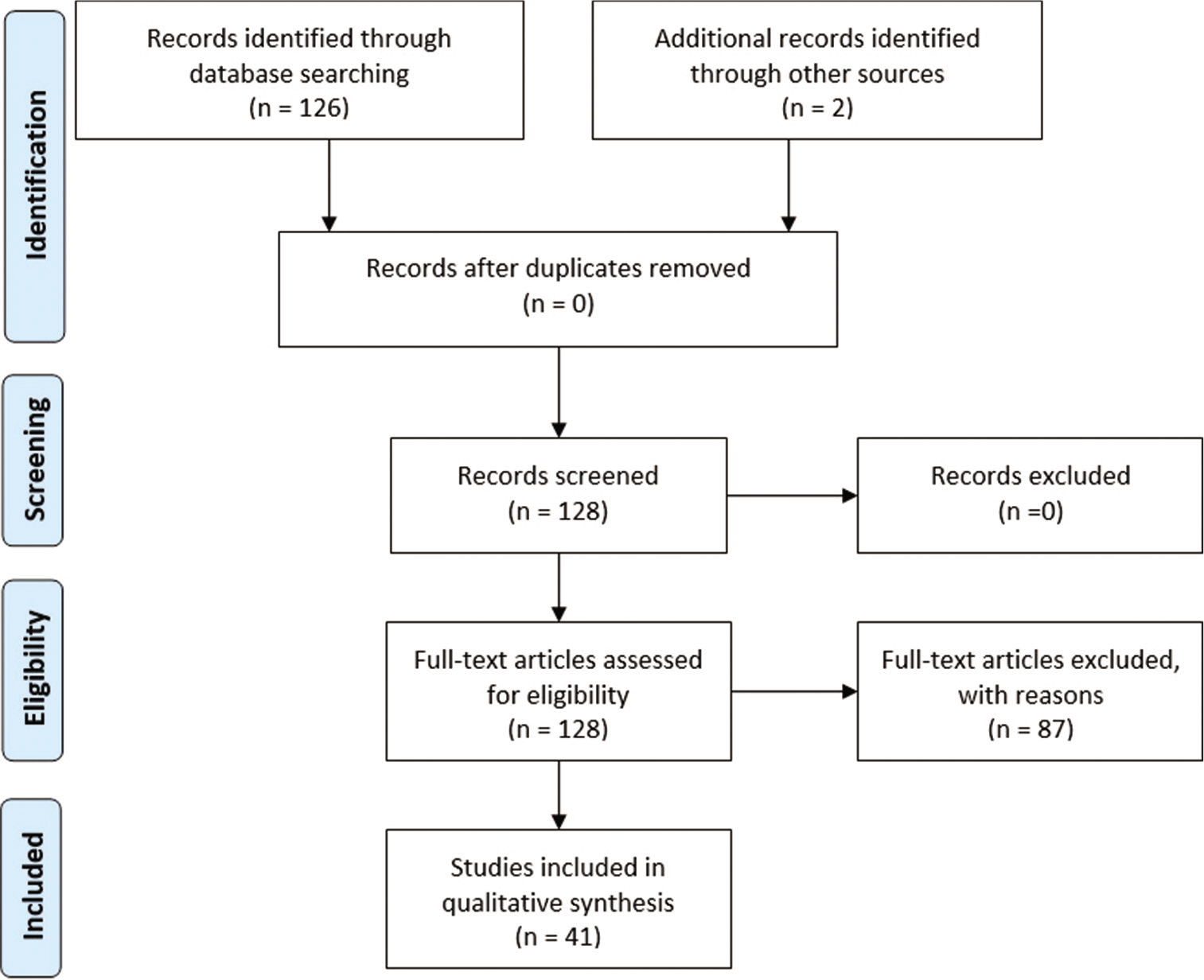
Results: A total of 128 published studies were identified through our search. 41 studies were included in this systematic review, 59 patients with a female/male ratio of 1.2/1. The mean age of the patients is of 47.69 years (range 3–84 years). Only seven out of 59 patients (11.9%) presented a complex intradiploic meningioma located in fronto-orbito-ethmoidal region like our case. In almost all patients, a gross-total resection was performed (96.6%) and only in two patients (3.4%) a subtotal resection was achieved.
Conclusion: The authors shared this successfully treated case to add to the overall clinical experience in the management of this rare subtype tumor, with the hope that more studies are conducted to further address the mechanism of intradiploic meningiomas development.
Keywords: Anterior skull base, Calvarial, Intradiploic, Intraosseous, Meningioma
INTRODUCTION
Meningiomas are one of the most common benign brain/intracranial tumors with an incidence rate of 15–20%.[
MATERIALS AND METHODS
The authors collected clinical, radiological, intraoperative, postoperative, and histological features of a patient harboring a rare case of left intradiploic fronto-orbito-ethmoidal meningioma. We also performed a systematic review in accordance with preferred reporting items for systematic reviews and meta-analysis guidelines [
CASE DESCRIPTION
History
A 47-year-old female patient was admitted to our department, following the progressive onset of left eye proptosis lasting for 4 years associated with occasional mild to moderate headache. She did not complain visual acuity decrease nor diplopia in any gaze direction. At the time of her first medical consultation (at another Institution), she was advised to perform an endoscopic trans-nasal biopsy; this procedure was then performed but the histology was not helpful (no more information about this surgical procedure is available). After this, she decided to have a second opinion with the aim of definitively cure her illness.
Physical examination
On physical examination, there was axial proptosis on the left side, diplopia, with a restriction of the extraocular movements in the superior and medial side. The patient also presented hyposmia. She suffered from an amblyopia dating back to the adolescence in the contralateral eye (visual acuity: 7/10); in the left eye acuity was 8/10. No visual fields defect was detected, and the fundus oculi examination was normal. Rest of the neurological examination was normal. Routine hematological and biochemical tests were normal.
Preoperative imaging
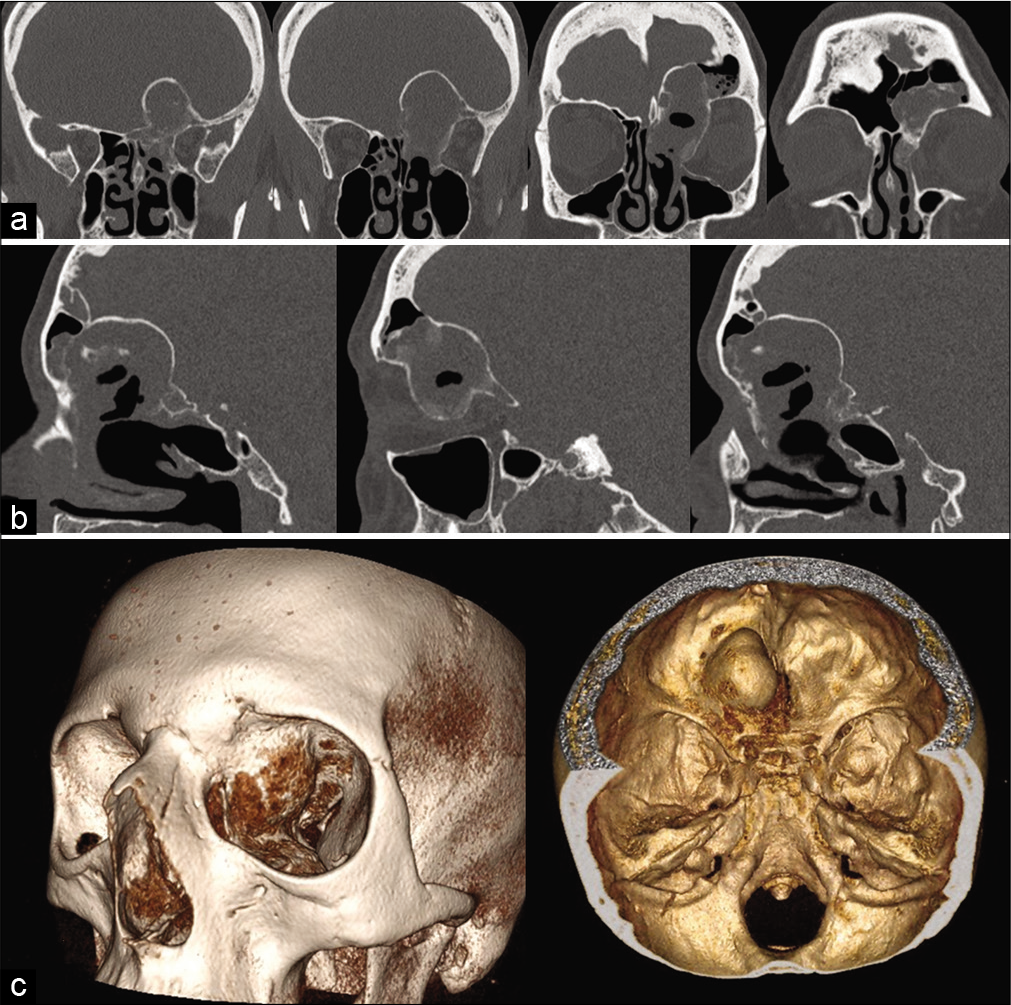
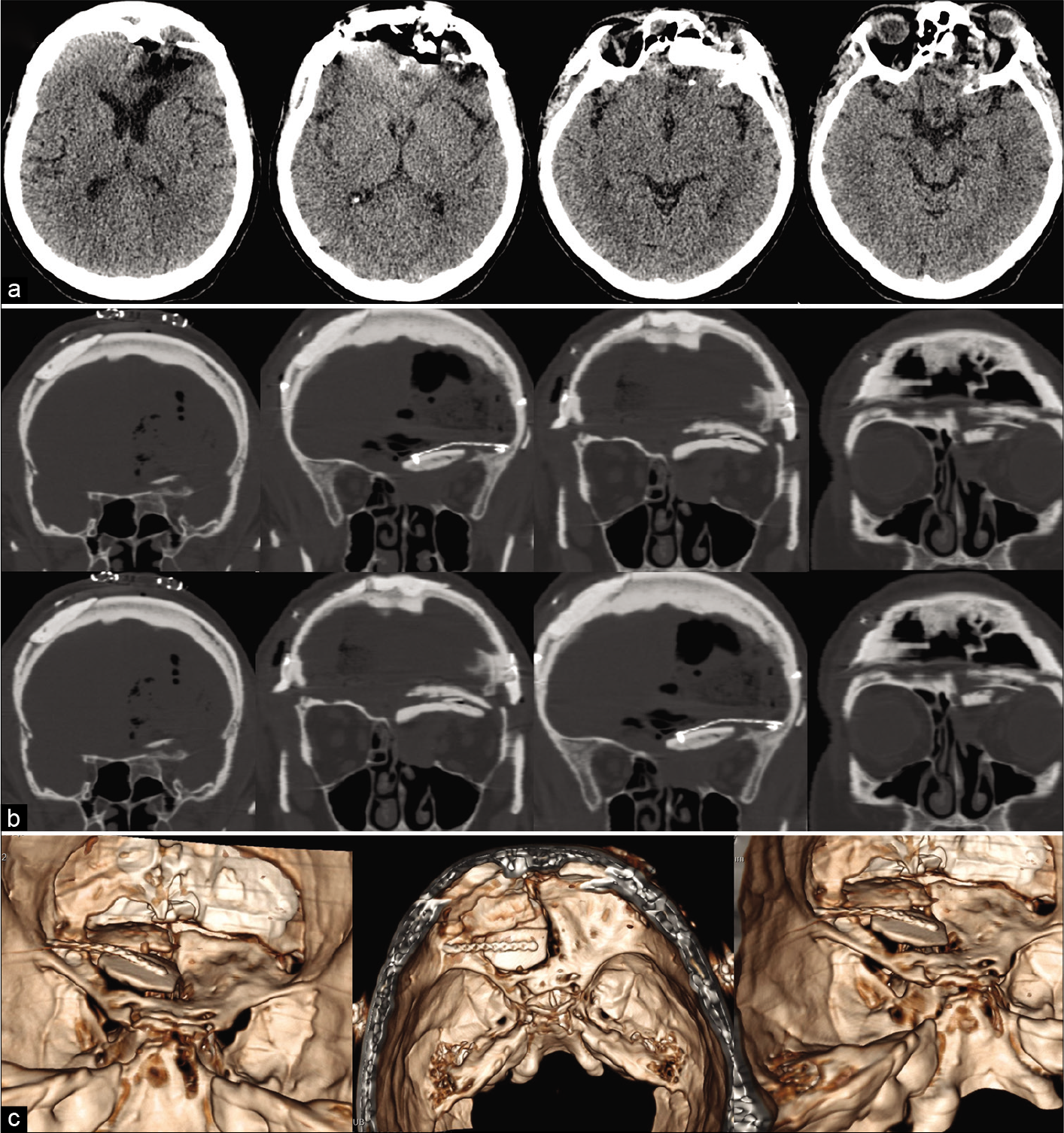
A brain computerized tomography (CT) scan with thin cuts of the orbit and 3D-reconstruction showed a roughly ovoidal lesion centered at the left medial wall and roof of the orbit and invading the left frontal sinus and left ethmoidal body bone. The lesion’s maximal diameter was about 5 cm, and it was surrounded by a heterogeneous bone shell filled internally by hypodense tissue with multiple sepimentations [
Surgical procedures
Neuronavigation was installed. A sterile surgical field was prepared at the level of the fascia lata to harvest a graft. A bicoronal skin incision with subcutaneous two-layer dissection (to save a vascularized pericranial flap) was performed. A bilateral fronto-basal transfrontal sinus craniotomy was done to expose the tumor. Tumor was found to be completely extra-axial destroying the orbital roof and the orbital medial wall and eroding the ethmoid. Bilateral frontal sinus was invaded by the lesion (especially in the left side). The dura of the left anterior skull base was massively elevated together with the frontal lobe and totally substituted by a thin layer of fibrous tissue. The lesion presented a hard consistence due to an external hyperostotic bone shell. At the inside there was a cavity filled with numerous sepimentations and blood clots. The periorbita was intact during the microsurgical dissection with a good cleavage plan. The tumor was removed through a piecemeal technique. Peripheral hyperostotic bone was drilled away to obtain a good control of the bleeding and an efficient removal. Frontal skull base dura was removed and reconstructed with watertight technique with a synthetic patch. Left olfactory nerve was encased in the mass and was not possible to be spared. The tumor was completely removed obtaining a Simpson I resection. Frontal and ethmoidal sinus was closed by free temporal muscle fragments, gel foam, and fibrin glue. Then, orbital roof, medial orbital wall, and ethmoid were reconstructed using the split internal cortical bone of the frontal flaps, to avoid any postoperative pulsatile proptosis. This reconstruction was then covered with fascia lata and fibrin glue. Finally, the whole anterior skull-base until the planum sphenoidale was covered and sealed up with a vascularized pericranial layer. The bony defects of the frontal craniotomy were corrected using a methylmethacrylate paste. A lumbar drain was placed to prevent any cerebrospinal fluid (CSF) leak (for a total of 8 days).
Histological examination
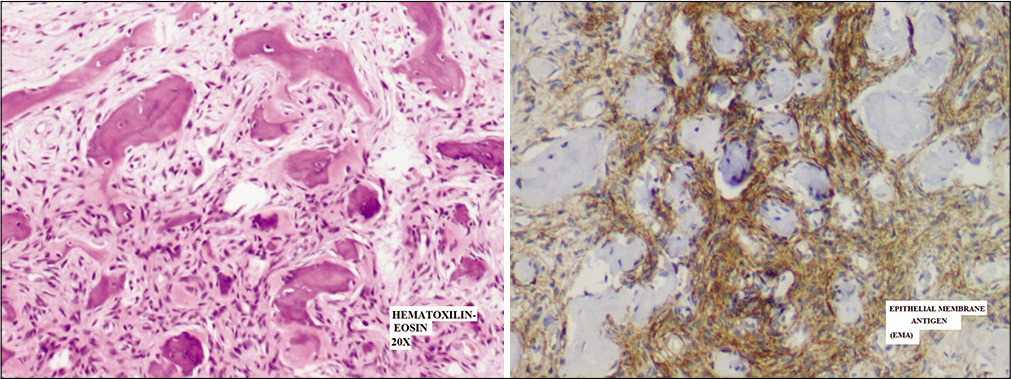
The histological analysis reported a metaplastic meningioma (the World Health Organization [WHO] Grade I) with extensive ossification with a bone angioectasia infiltration (immunostaining showed Epithelial Membrane Antigen +, Vimentine +, S100-, CKpool -, CD4-, Progesterone -) [
Figure 4:
Routine histology showing neoplastic proliferation of medium-sized spindle tumor cells with no atypia, forming fascicles in metaplastic bone tissue and spreading in normal trabecular bone (up); neoplastic cells with epithelial membrane antigen immunoreactivity (Epithelial Membrane Antigen) (down).
Postoperative course and postoperative imaging
At the recovery from the general anesthesia, the patient was drowsy and strongly apathetic. CT scan demonstrated a contusion and reactive edema at the basal frontal lobe. Intensive care stay was prolonged, and patient gradually recovered to a satisfying state of consciousness after eight days. Postoperative CT scan showed a total removal of the lesion, with an optimal reconstruction of the medial wall and the roof of the left orbit, in the absence of complications [
Figure 5:
Postoperative axial head computed tomography scan images in series (a), with bone algorithm (b) and with 3D-reconstruction (c) showing a proper reconstruction of the left orbital roof and the medial orbital wall with split calvarial bone graft and bone defect reconstruction with methyl-methacrylate paste, obtaining good cosmetic and functional results.
Late rhinoliquorrhea management
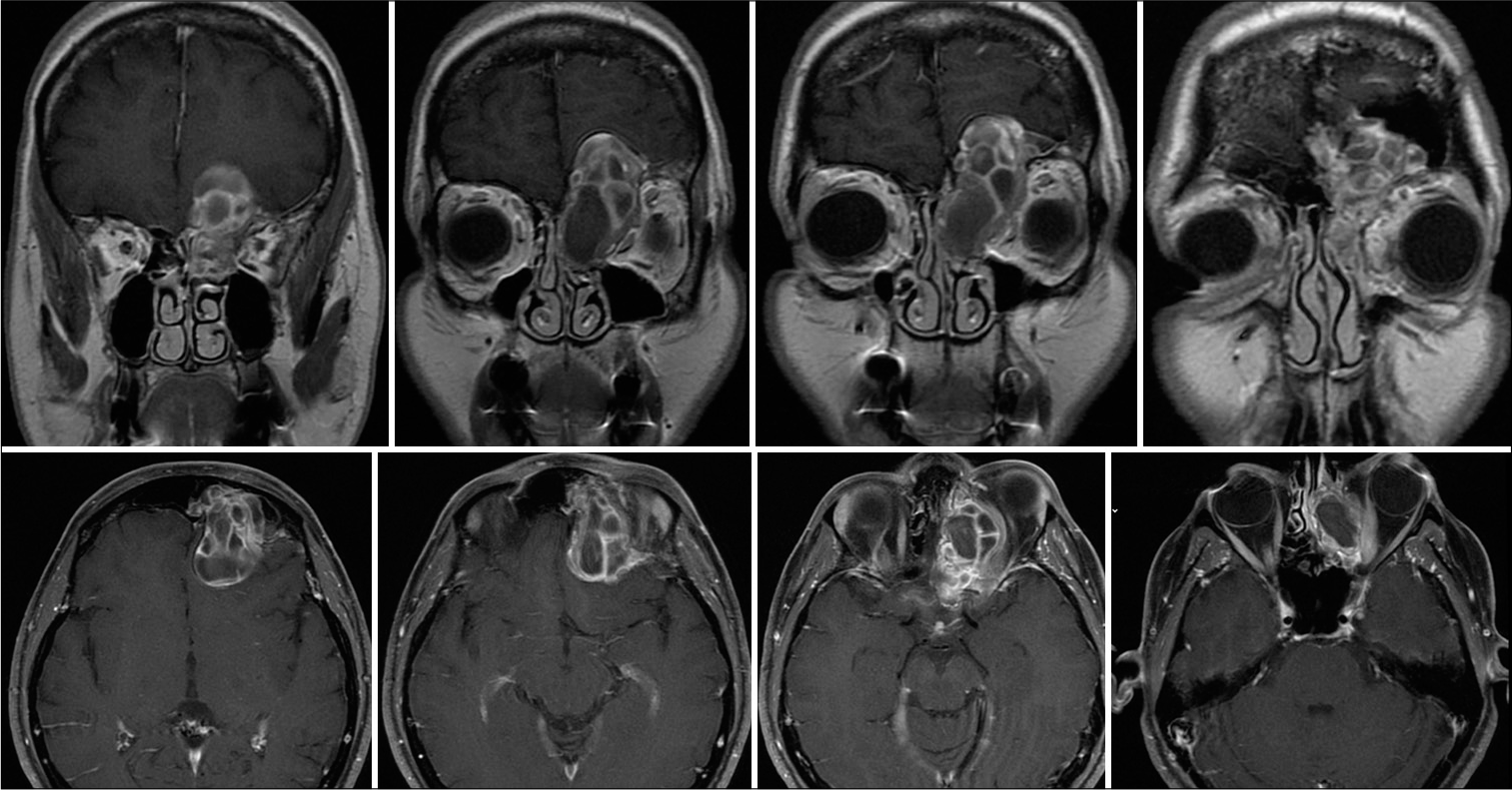
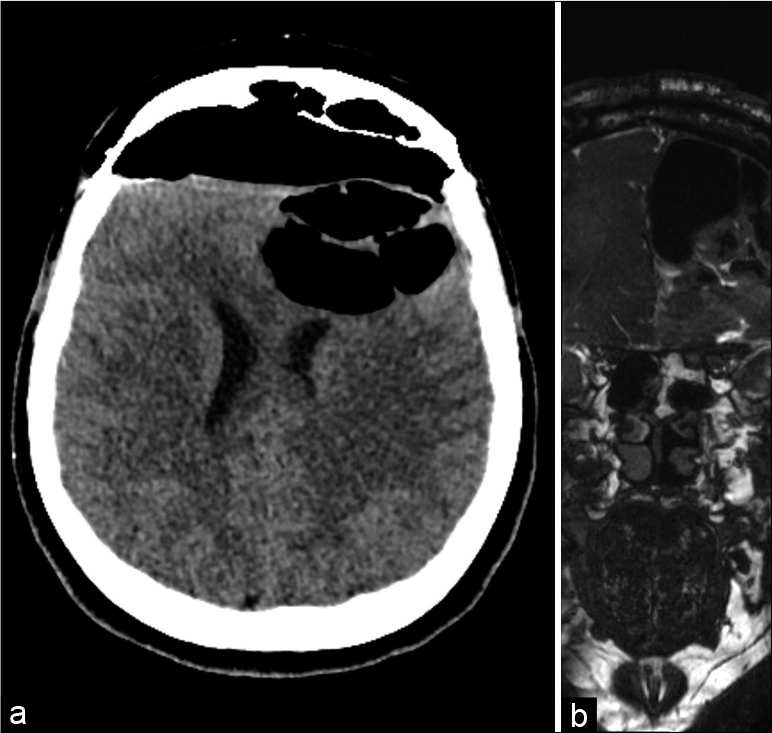
About 3 months from the previous CT scan control, the patient presented to the local emergency room for acute recurrence of rhinoliquorrhea. According to the COVID-19 re-organization model adopted by the Lombardy region, the patient was transferred to the neurosurgical reference hub in the area. When she arrived at the Neurosurgical department of the “Spedali Civili” Hospital in Brescia, the patient underwent a brain CT and MRI that showed the presence of pneumocephalus with a small defect in the posterior ethmoidal plane close to the spheno-ethmoidal suture with pneumocephalus [
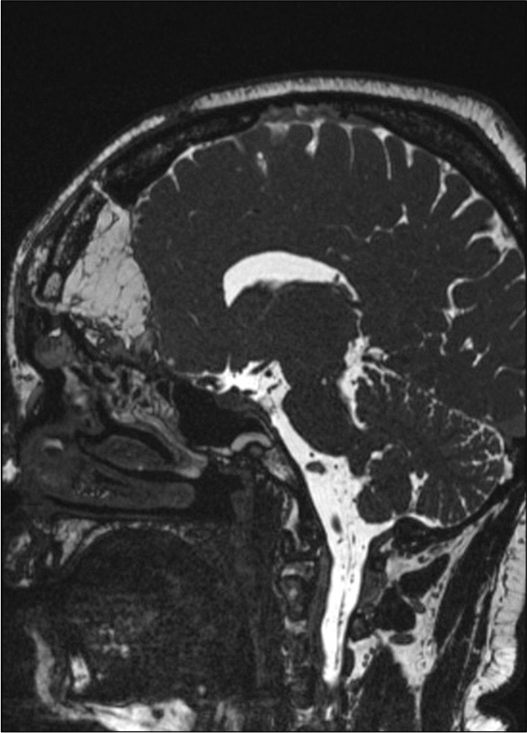
Silicone coated pads were positioned to make the flap adhere better to hold the construct (the endonasal swabs will then be removed 4 days after surgery). The frontal bone operculum was positioned and fixed with plates and screw (Synthes Matrix). Postoperative course was uneventful. A control head CT scan showed good surgical results and resolution of the pneumocephalus. An endoscopic exploration showed good vitality and adhesion of the mucosal flap. After prophylactic therapy (vancomycin + cephalosporin for 10 days), the patient was discharged at home neurologically intact. The 3-month postoperative MRI showed no residual or recurrent disease [
RESULTS
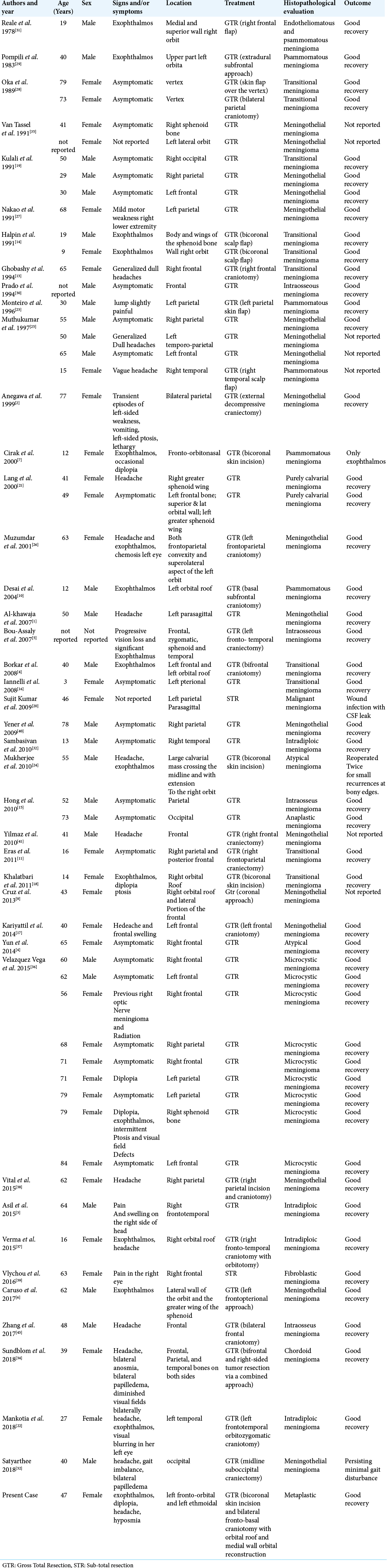
A total of 128 published studies were identified through our detailed searches from 1955 to 2021. After a detailed examination of the titles, abstracts, and contents of these studies, 87 were rejected from our systematic review because they were not in English language, not purely intradiploic calvarial meningiomas, or did not include accurate data. Our case was not included in this systematic review. A summary of patients’ characteristics in selected papers are reported in [
DISCUSSION
A localization of meningiomatous tissue at the level of the orbita, it is most often due to secondary invasion of the orbit or of the orbital walls by a tumor starting from the meninges enveloping the brain, especially at the level of the anterior cranial fossa or of the sphenoid lesser wing.[
Intradiploic meningiomas of the orbital roof are more frequent in males and in young people.[
X-ray, head CT, and brain MRI are methods of radiological screening in diagnosing intradiploic meningioma. Radiologically intradiploic meningiomas are typically either osteoplastic or osteolytic, and although the majority are osteoblastic, extremely rarely are osteolytic or very extremely have a mixed pattern. These can present with opacity of the orbital roof on X-ray skull study. The osteolytic subtype of intradiploic meningiomas is more likely to be malignant than the osteoblastic subtype. Intradiploic meningiomas should be considered in the differential diagnosis of patients presenting with osteoblastic or osteolytic skull lesions.[
Tumor excision with wide surgical resection and meticulous bony reconstruction is the primary choice for treatment in symptomatic patients[
CONCLUSION
Primary intradiploic meningiomas are rare tumors, which are used to describe the subset of extradural meningiomas that arise in the skull. To the best of our knowledge, only seven cases of complex intradiploic fronto-orbito-ethmoidal meningioma were reported in the literature. Regular monitoring is essential in the management of intradiploic meningioma and surgical treatment with wide incision of the lesion is the goal of the primary therapy. We share this successfully treated case to add to the overall clinical experience in the management of this rare subtype tumor, with the hope that more studies are conducted to further address the mechanism of intradiploic meningiomas development.
Ethical approval
All procedures in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Ethical approval
All procedures in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Professor Francesco Doglietto for the surgical management and supervision of the clinical case.
References
1. Al-khawaja D, Murali R, Sindler P. Primary calvarial meningioma. Case reports. J Clin Neurosci. 2007. 14: 1235-9
2. Anegawa S, Hayashi T, Torigoe R. Diffuse calvarial meningioma. J Neurosurg. 1999. 90: 970-3
3. Asil K, Aksoy YE, Yaldiz C. Primary intraosseous meningioma mimicking osteosarcoma: Case report. Turk Neurosurg. 2015. 25: 174-6
4. Borkar SA, Tripathi AK, Satyarthee GD. Fronto-orbital intradiploic transitional meningioma. Neurol India. 2008. 56: 205-6
5. Bou-Assaly W, Illner A, Mosier KM. Intra-osseous meningioma of the orbit: An unusual presentation. Eur Radiol. 2007. 17: 2192-4
6. Caruso R, Fini G, Pesce A. A primary intraosseous cystic meningioma: Case report. Int J Surg Case Rep. 2017. 37: 189-92
7. Cirak B, Guven MB, Ugras S. Fronto-Orbitonasal intradiploic meningioma in a child. Pediatr Neurosurg. 2000. 32: 48-51
8. Craig WM, Gogela LJ. Meningioma of the optic foramen as a cause of slowly progressive blindness: Report of three cases. J Neurosurg. 1950. 7: 44-8
9. Cruz AA, Rota LM, Trevenzol FP. Hyperplastic intradiploic meningiothelial tissue in the orbital roof mimicking metastatic disease. Ophthal Plast Reconstr Surg. 2013. 29: 1
10. Desai KI, Nadkarni TD, Bhayani RD. Intradiploic meningioma of the orbit: A case report. Neurol India. 2004. 52: 380-2
11. Eras MA, Tari R, Ozturk G. Intraosseous meningioma mimicking osteosarcoma in an adolescent: A case report. Turk Neurosurg. 2011. 21: 663-5
12. Germano IM, Edwards MS, Davis RL. Intracranial meningiomas of the first two decades of life. J Neurosurg. 1994. 80: 447-53
13. Ghobashy A, Tobler W. Intraosseous calvarial meningioma of the skull presenting as a solitary osteolytic skull lesion: Case report and review of the literature. Acta Neurochir (Wien). 1994. 129: 105-8
14. Halpin SF, Britton J, Wilkins P. Intradiploic meningiomas. A radiological study of two cases confirmed histologically. Neuroradiology. 1991. 33: 247-50
15. Hong B, Hermann EJ, Klein R. Surgical resection of osteolytic calvarial lesions: clinicopathological features. Clin Neurol Neurosurg. 2010. 112: 865-9
16. Iannelli A, Pieracci N, Bianchi MC. Primary intra-diploic meningioma in a child. Childs Nerv Syst. 2008. 24: 7-11
17. Kariyattil R, Govindaraju V, Hamid RS. Primary extradural meningioma presenting as frontal sinusitis with extensive bony changes: Case report. SQU Med J. 2014. 14: e566-70
18. Khalatbari MR, Borghei-Razavi H, Moharamzad Y. Intradiploic orbital roof meningioma with pneumosinus dilatans in a child: A case report and review of the literature. Pediatr Neurosurg. 2011. 47: 66-71
19. Kulali A, Ilçayto R, Rahmanli O. Primary calvarial ectopic meningiomas. Neurochirurgica (Stuttg). 1991. 34: 174-7
20. Kumar GS, Chacko G, Chacko AG. Multiple extracranial metastases from intradiploic meningioma. Neurol India. 2009. 57: 96-7
21. Lang FF, Kenneth Macdonald O, Fuller GN. Primary extradural meningiomas: A report on nine cases and review of the literature from the era of computerized tomography scanning. J Neurosurg. 2000. 93: 940-50
22. Mankotia DS, Singh SK, Borkar SA. Primary giant spheno temporal intradiploic meningioma. Asian J Neurosurg. 2018. 13: 157-60
23. Monteiro JT, Baptista AE, Trabulo AS. Intradiploic meningioma of the skull: Case report and review of the literature. Neurocirugìa. 1996. 7: 129-32
24. Mukherjee KK, Salunke P, Chhabra R. Temporary bilateral external carotid artery clamping to reduce blood loss during removal of calvarial meningioma: A technical note. Br J Neurosurg. 2011. 25: 127-9
25. Muthukumar N. Primary calvarial meningiomas. Br J Neurosurg. 1997. 11: 388-92
26. Muzumdar DP, Vengsarkar US, Bhatjiwale MG. Diffuse calvarial meningioma: A case report. J Postgrad Med. 2001. 47: 116-8
27. Nakao N, Kubo K, Moriwaki H. Multiple growths of primary calvarial meningiomas. Neurosurgery. 1991. 29: 3
28. Oka K, Hirakawa K, Yoshida S. Primary calvarial meningiomas. Surg Neurol. 1989. 32: 304-10
29. Pompili A, Caroli F, Cattani F. Intradiploic meningioma of the orbital roof. Neurosurgery. 1983. 12: 5
30. Prado JA, Sener RN, Jinkins JR. Primary intraosseous calvarial meningioma. Comput Med Imaging Graphics. 1994. 18: 467-8
31. Reale F, Delfini R, Cintorino M. An intradiploic meningioma of the orbital roof: Case report. Ophtalmologica. 1978. 177: 82-7
32. Sambasivan M, Kumar PS, Mahesh S. Primary intradiploic meningioma in the pediatric age-group. J Pediatr Neurosci. 2010. 5: 76-8
33. Satyarthee GD. Primary extradural meningioma of posterior fossa associated with acquired chiari malformation: A short review. Asian J Neurosurg. 2018. 13: 421-4
34. Sundblom J, Nowinski D, Casar-Borota O. Removal of giant intraosseous meningioma followed by cranioplasty using a custom-made bioceramic implant: Case report. J Neurosurg. 2018. 131: 735-9
35. Van Tassel P, Lee YY, Ayala A. Case report 680. Skeletal Radiol. 1991. 20: 383-6
36. Vega JE, Rosenberg A. Microcystic meningioma of the calvarium: A series of 9 cases and review of the literature. Am J Surg Pathol. 2015. 39: 4
37. Verma SK, Satyarthee G, Borkar SA. Orbital roof intradiploic meningioma in a 16-year-old girl. J Pediatr Neurosci. 2015. 10: 51-4
38. Vital RB, Filho PT, Lapate RL. Calvarial ectopic meningothelial meningioma. Int J Surg Case Rep. 2015. 10: 69-72
39. Vlychou M, Inagaki Y, Stacey R. Primary intraosseous meningioma: An osteosclerotic bone tumour mimicking malignancy. Clin Sarcoma Res. 2016. 6: 14
40. Yener U, Bayrakli F, Vardereli E. Intradiploic meningioma mimicking calvarial metastasis: Case report. Turk Neurosurg. 2009. 19: 297-301
41. Yilmaz A, Musluman M, Aydin Y. Primary osteolytic intraosseous meningioma of the frontal bone. Neurol I Neurochirurg Polska. 2010. 44: 415-8
42. Yun JH, Lee SK. Primary osteolytic intraosseous atypical meningioma with soft tissue and dural invasion: Report of a case and review of literatures. J Korean Neurosurg Soc. 2014. 56: 509-12
43. Zhang S, Zhang J, Chen J. Frontal intradiploic meningioma with progressive intracranial invasion. Medicine. 2017. 96: 34