- Department of Neurosurgery, Yamagata City Hospital Saiseikan, Yamagata, Japan.
- Department of Emergency Medicine, Yamagata City Hospital Saiseikan, Yamagata, Japan.
- Department of Neurosurgery, Yamagata University, Yamagata, Japan.
Correspondence Address:
Atsushi Kuge, Department of Neurosurgery and Emergency Medicine, Yamagata City Hospital Saiseikan, Yamagata, Japan.
DOI:10.25259/SNI_695_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kosuke Sasaki1, Atsushi Kuge1,2, Yu Shimokawa1, Tetsu Yamaki1, Rei Kondo1, Yukihiko Sonoda3. Intracranial parenchymal capillary hemangioma: A case report. 17-Nov-2023;14:401
How to cite this URL: Kosuke Sasaki1, Atsushi Kuge1,2, Yu Shimokawa1, Tetsu Yamaki1, Rei Kondo1, Yukihiko Sonoda3. Intracranial parenchymal capillary hemangioma: A case report. 17-Nov-2023;14:401. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12639
Abstract
Background: Capillary hemangioma is a rare benign hemangioma that occurs in the soft tissues of the skin, orbit, head, and neck. Intracranial cases, especially intraparenchymal cases, are extremely rare. In this study, we report the course of an intracranial parenchymal capillary hemangioma with left mild motor paresis and involuntary movements of the left upper extremity and was successfully treated by surgical resection, including radiological and pathological examinations.
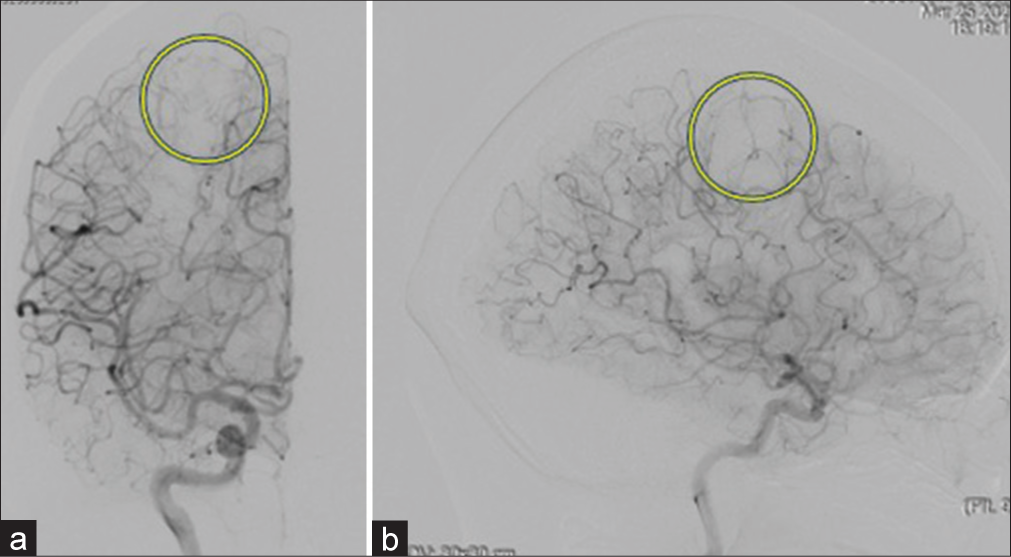
Case Description: This is a case of a 60-year-old woman who presented with motor weakness and involuntary movement of the left upper extremity. Computed tomography and magnetic resonance imaging revealed the right frontal hemorrhagic mass lesion without enhancement of contrast medium. Cerebral digital subtraction angiography showed no vascular stain and abnormal arteriovenous shunt. Preoperatively, we diagnosed cavernous hemangioma with a hemorrhagic component located in the right motor cortex. Because this case was symptomatic, we performed a craniotomy and gross total resection of the right frontal lesion. The diagnosis of capillary hemangioma was made by histological examination, including immunohistological study.
Conclusion: Because intraparenchymal capillary hemangiomas are difficult to diagnose with preoperative imaging, surgical treatment, and histopathological examination are important.
Keywords: Capillary hemangioma, Intraparenchymal, Pathological findings, Radiological findings
INTRODUCTION
Capillary hemangioma is an uncommon, benign vascular neoplasm that manifests within the subcutaneous tissues of the integumentary system, orbit, cranium, and cervicocephalic region. The reported incidence of this condition in neonates ranges from 1.1% to 2.6%, but intracranial presentations are exceedingly rare occurrences.[
CASE REPORT
A 60-year-old female patient with no remarkable medical history was admitted to our hospital due to the presence of involuntary movements and weakness in her left upper extremity. Upon examination, her level of consciousness was Glasgow Coma Scale: E4V5M6, and she exhibited weakness, specifically in her left upper extremity. A computed tomography (CT) scan revealed a high-density area (3 mL) located in the right motor cortex, suggesting subcortical hemorrhage [
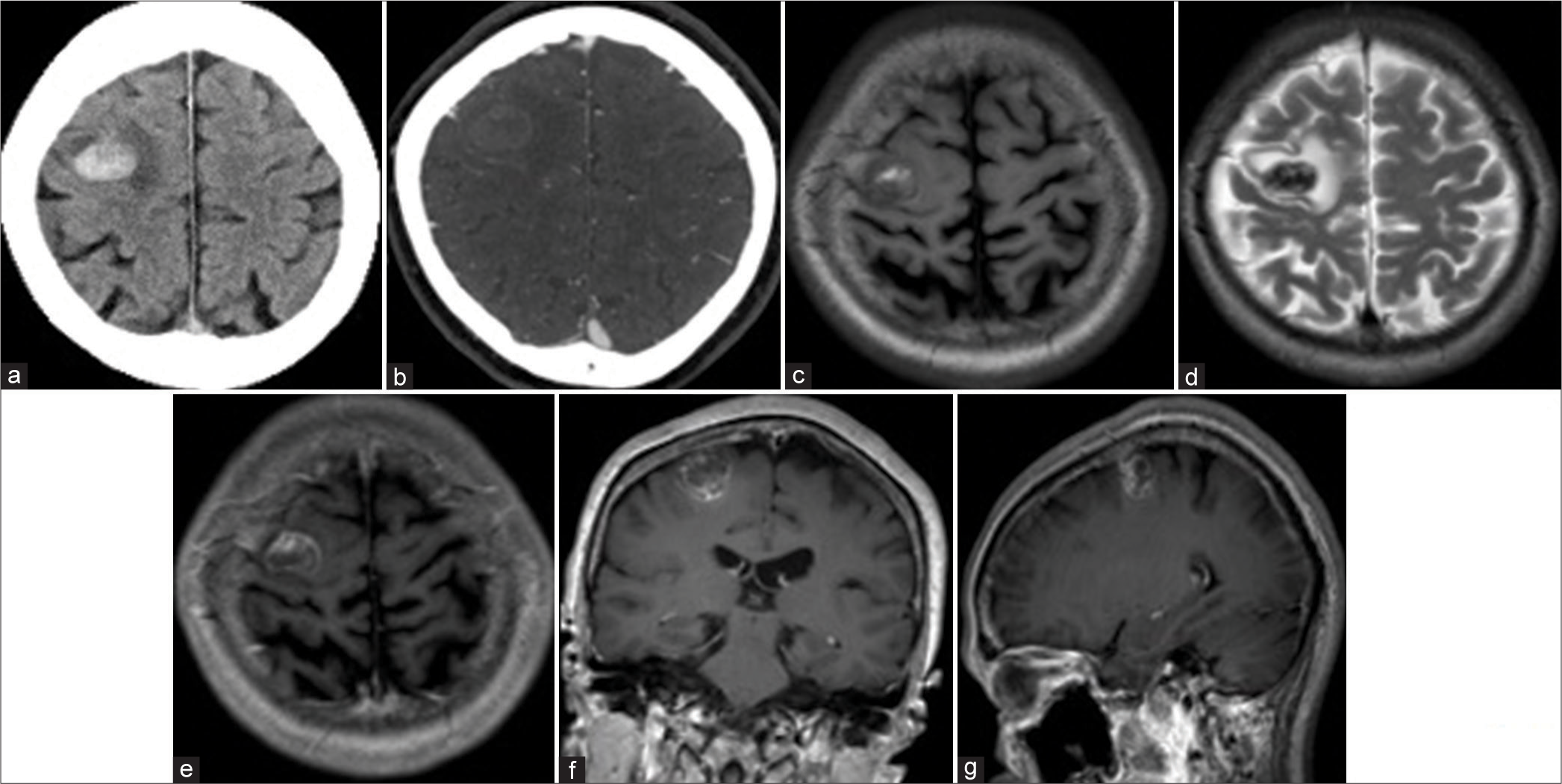
Figure 1:
Head computed tomography (CT) and brain magnetic resonance imaging (MRI). (a and b) CT showed that a high-density lesion in the right precentral gyrus indicates intraparenchymal hemorrhage without contrast enhancement. (c and d) MRI T1 and T2-weighted images showed that heterogeneous intensity mass lesion at the precentral gyrus consists of hemorrhagic change. (e-g) MRI T1-weighted image with gadolinium (e: axial view, f: coronal view, g: sagittal view) exhibited a heterogeneous intensity mass with surrounding contrast enhancement. There was no flow void around the lesion.
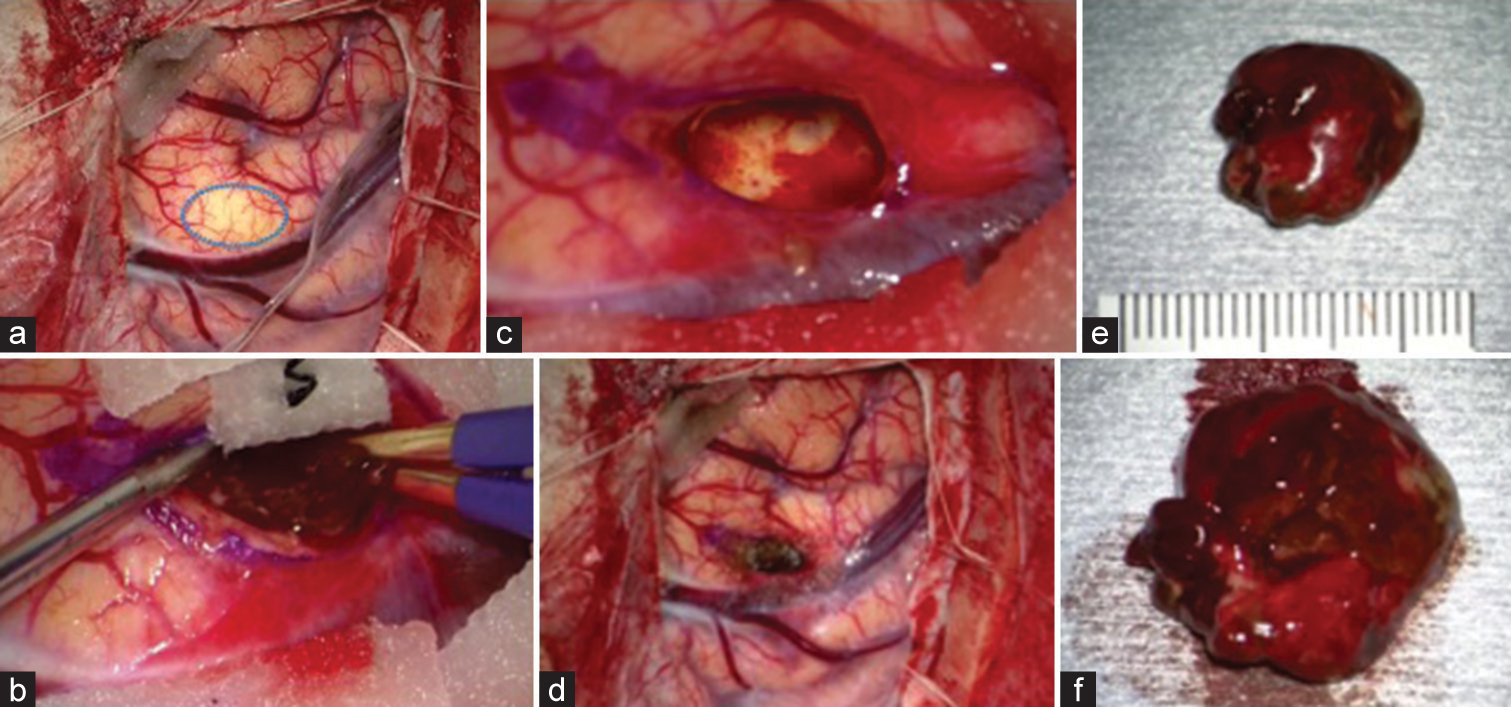
Figure 3:
Intraoperative findings. (a) Upon exposure of the cortex, edema, and swelling were observed, and the brain surface was pale above the lesion (blue circle). (b-d) En-bloc resection was performed. We could see a nonencapsulated mass lesion with hemorrhagic change (b), and the border between the white matter and the lesion was well-defined (c). (e and f) The specimen exhibited a reddish-black color; upon inspection of the lesion, no mulberry formations were identified, suggesting that this lesion might differ from cavernous hemangioma.
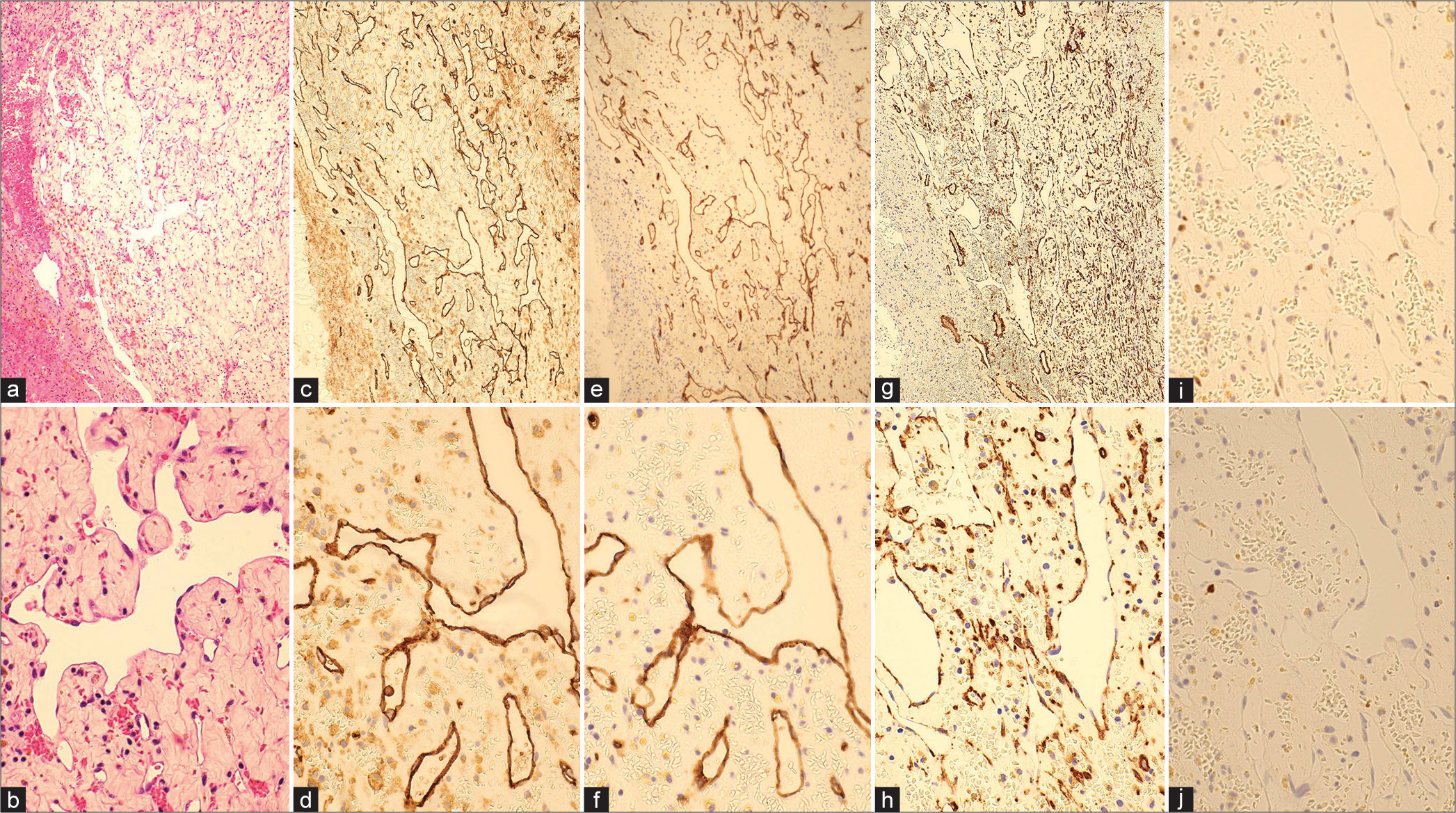
Figure 4:
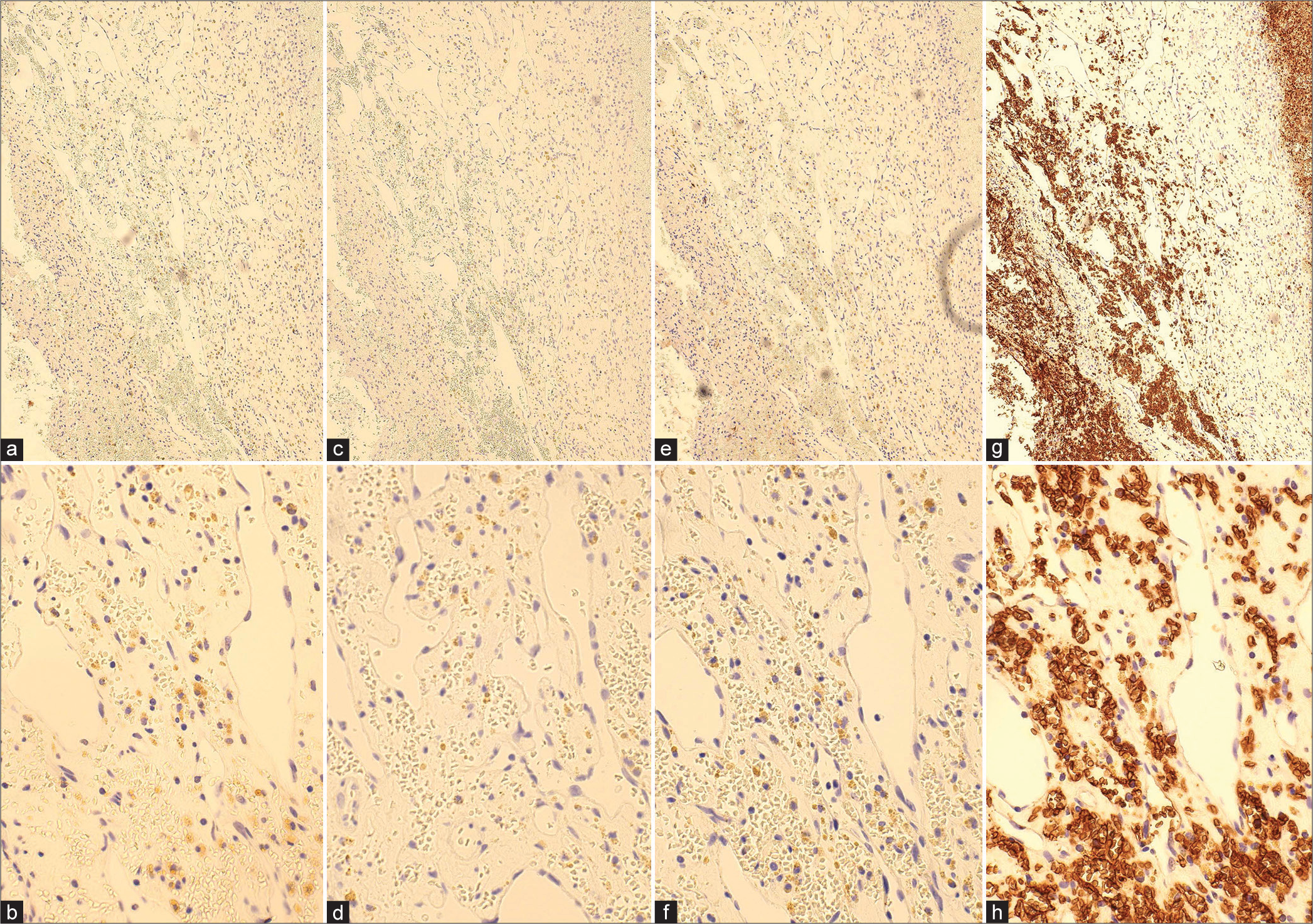
Micrographs of hematoxylin-eosin (HE) and immunohistochemical findings (CD 31, CD 34, SMA, Ki-67, p53). (a and b) HE staining ×10, ×40: capillary-like vascular structures with thin-walled endothelial cells displaying irregular anastomoses. There was no blood filling within the vessels as seen in the histopathology of cavernous hemangiomas, and the extravascular area consisted of thick connective tissue; (c and d) immunohistochemical staining: CD 31 ×10, ×40; (e and f) immunohistochemical staining: CD 34 ×10, ×40; (g and h) immunohistochemical staining: SMA ×10, ×40; (i) p53; (j) Ki-67. Immunostaining demonstrated positive expression of CD31 and CD34, indicative of endothelial cells forming irregular lumens.
DISCUSSION
Capillary hemangioma represents the most prevalent benign vascular neoplasm affecting the integumentary system and mucous membranes. Typically observed in neonates, the majority of these lesions undergo spontaneous regression by the age of 5 years.[
Although regression of residual lesions has been reported following systemic administration of steroids and interferon alpha, surgical excision represents the most common treatment approach. Hemorrhage from partially excised lesions has been documented in some cases, so total resection of the tumor was recommended.[
CONCLUSION
Capillary hemangioma within cerebral parenchyma is very rare, especially in adults. For symptomatic lesions, surgical treatment is a valid option for histopathological characterization and neurological improvement. The etiology of intraparenchymal capillary hemangioma has not been established, and more cases need to be accumulated.
Authors contributions
Kosuke Sasaki: Writing and editing; Atsushi Kuge (Corresponding author): Conceptualization, editing; Yu Shimokawa: Data curation, investigation; Tetsu Yamaki : Data curation, investigation; Rei Kondo: Methodology, editing; Yukihiko Sonoda: Supervision.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abe M, Tabuchi K, Tanaka S, Hodozuka A, Kunishio K, Kubo N. Capillary hemangioma of the central nervous system. J Neurosurg. 2004. 101: 73-81
2. Abe M, Misago N, Tanaka S, Masuoka J, Tabuchi K. Capillary hemangioma of the central nervous system: A comparative study with lobular capillary hemangioma of the skin. Acta Neueopathol. 2005. 109: 151-8
3. Daenekindt T, Weyns F, Kho KH, Peuskens D, Engelborghs K, Wuyts J. Giant intracranial capillary hemangioma associated with enlarged head circumference in a newborn. J Neurosurg Pediatr. 2008. 1: 488-92
4. Ishikawa T, Takeuchi K, Nagata Y, Ito K, Yamamoto T, Kabeya R. Case of pregnant woman with capillary hemangioma of the parasellar region. NMC Case Rep J. 2022. 9: 77-82
5. John SG, Pillai U, Lacasse A. Intracranial capillary hemangioma mimicking a dissociative disorder. Clin Pract. 2012. 24: e35
6. Karikari IO, Selznick LA, Cummings TJ, George TM. Capillary hemangioma of the fourth ventricle in an infant. J Neurosurg. 2006. 104: 188-191
7. Koga Y, Hamada S, Saito H, Akai T, Kuroda S. Intracranial, intra-parenchymal capillary hemangioma-case report. NMC Case Rep J. 2020. 7: 43-6
8. Santoro G, Piccirilli M, Chiarella V, Greco N, Berra LV, Santoro A. Intracranial capillary hemangiomas: Literature review in pediatric and adult population. Neurosurg Rev. 2021. 44: 1977-85
9. Simon SL, Moonis G, Judkins AR, Scobie J, Burnett MG, Riina HA. Intracranial capillary hemangioma: Case report and review of the literature. Surg Neurol. 2005. 64: 154-9
10. Younas F, Durrani Q, Shahzad MA, Mushtaq A, Imlay S. Multiple intracranial capillary hemangiomas and transient cerebrovascular insufficiency. Neurol Sci. 2011. 32: 963-6