- Department of Neurosurgery, Nagoya University Graduate School of Medicine,
- Division of Molecular Oncology, Aichi Cancer Center Research Institute,
- Department of Neurosurgery, Nagoya Central Hospital,
- Nagoya Radiosurgery Center, Nagoya Kyoritsu Hospital,
- Department of Pathology and Laboratory Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan,
- Department of Respiratory Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Correspondence Address:
Dr. Kuniaki Tanahashi, Department of Neurosurgery, Nagoya University Graduate School of Medicine, Nagoya, Japan.
DOI:10.25259/SNI_264_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kuniaki Tanahashi1, Masaki Hirano1,2, Lushun Chalise3, Takahiko Tsugawa4, Yuka Okumura5, Tetsunari Hase6, Fumiharu Ohka1, Kazuya Motomura1, Kazuhito Takeuchi1, Yuichi Nagata1, Norimoto Nakahara3, Naozumi Hashimoto6, Ryuta Saito1. 11C-methionine- and 18F-FDG-PET double-negative metastatic brain tumor from lung adenocarcinoma with paradoxical high 18F-FDG uptake: A case report. 19-Aug-2022;13:372
How to cite this URL: Kuniaki Tanahashi1, Masaki Hirano1,2, Lushun Chalise3, Takahiko Tsugawa4, Yuka Okumura5, Tetsunari Hase6, Fumiharu Ohka1, Kazuya Motomura1, Kazuhito Takeuchi1, Yuichi Nagata1, Norimoto Nakahara3, Naozumi Hashimoto6, Ryuta Saito1. 11C-methionine- and 18F-FDG-PET double-negative metastatic brain tumor from lung adenocarcinoma with paradoxical high 18F-FDG uptake: A case report. 19-Aug-2022;13:372. Available from: https://surgicalneurologyint.com/surgicalint-articles/11804/
Abstract
Background: Imaging with 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) and 11C-methionine (MET)-PET can delineate primary and metastatic brain tumors. Lesion size affects the sensitivity of both scans and histopathological features can also influence FDG-PET, but the effects on MET-PET have not been elucidated.
Case Description: We report an unusual case of metastatic brain tumors without accumulation of FDG or MET, contrasting with high FDG uptake in the primary lung lesion. The brain lesions were identified as adenocarcinoma with a more mucus-rich background, contributing to the indistinct accumulation of both FDG and MET.
Conclusion: Histopathological characteristics can affect both MET and FDG accumulation, leading to findings contradicting those of the primary lesion.
Keywords: 11C-methionine-PET, 18F-fluorodeoxyglucose-PET, Lung adenocarcinoma, Metastatic brain tumor, Mucus
INTRODUCTION
Tissue metabolism can be reflected by 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) and 11C-methionine (MET)-PET, making these imaging modalities useful for delineating and assessing both primary and metastatic brain tumors.[
CASE DESCRIPTION
A 63-year-old man with the left deafness due to a small left acoustic neurinoma presented with a 1-month history of dizziness and gait disorder. Contrast-enhanced magnetic resonance imaging (MRI) demonstrated multiple tumors in the right cerebellar hemisphere, right cerebellar peduncle, and right parietal lobe [
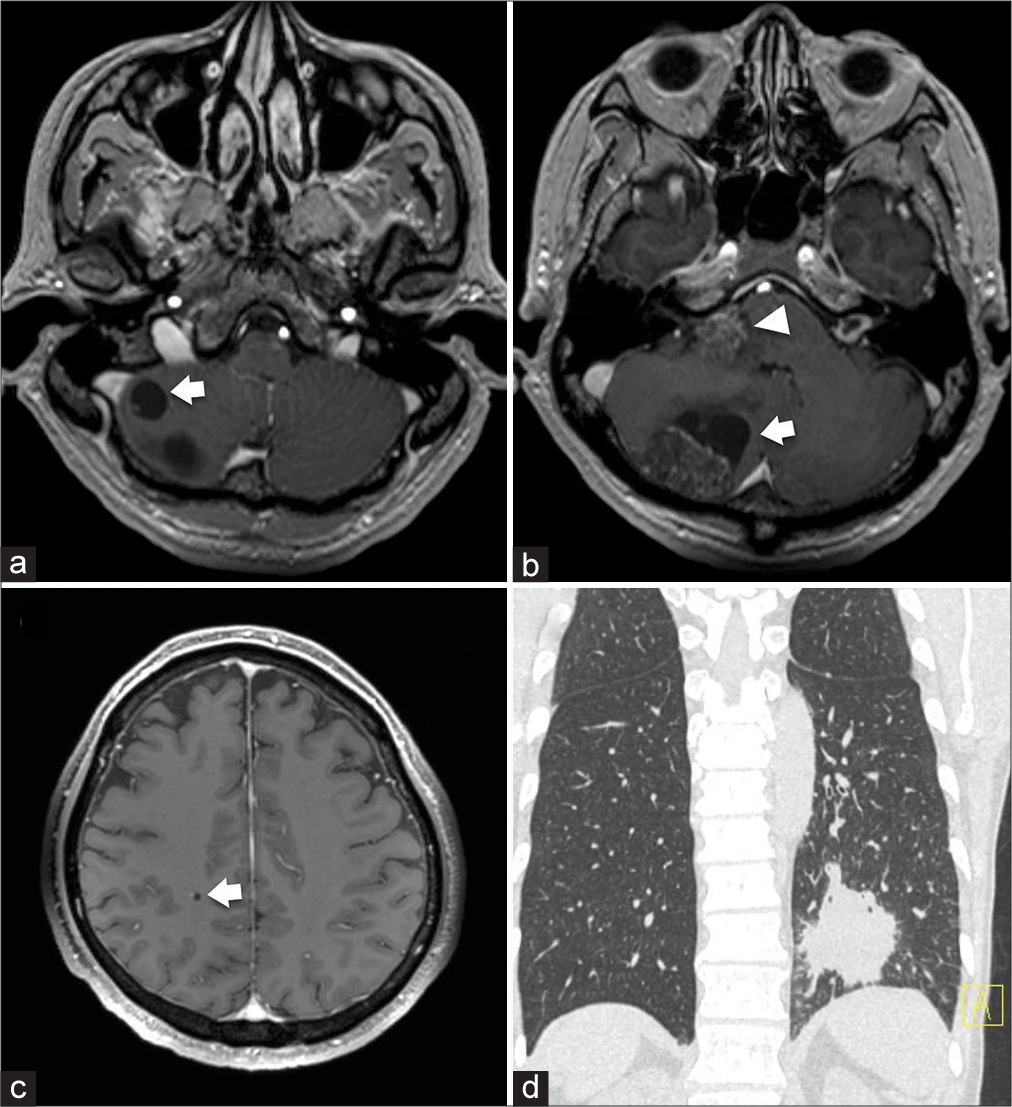
Figure 1:
(a-c) Gadolinium-enhanced magnetic resonance imaging of the brain at the initial visit. Two enhanced lesions are apparent in the right cerebellar hemisphere (31 × 19-mm solid component with 24 × 14-mm cystic part; 10 × 10-mm cystic tumor, arrows), one in the right cerebellar peduncle (22 × 18 mm, arrowhead) (a and b), and one in the right parietal lobe (5 × 4 mm, arrow) (c). (d) Computed tomography of the lung at the initial visit. A mass lesion is seen in the inferior lobe of the left lung.
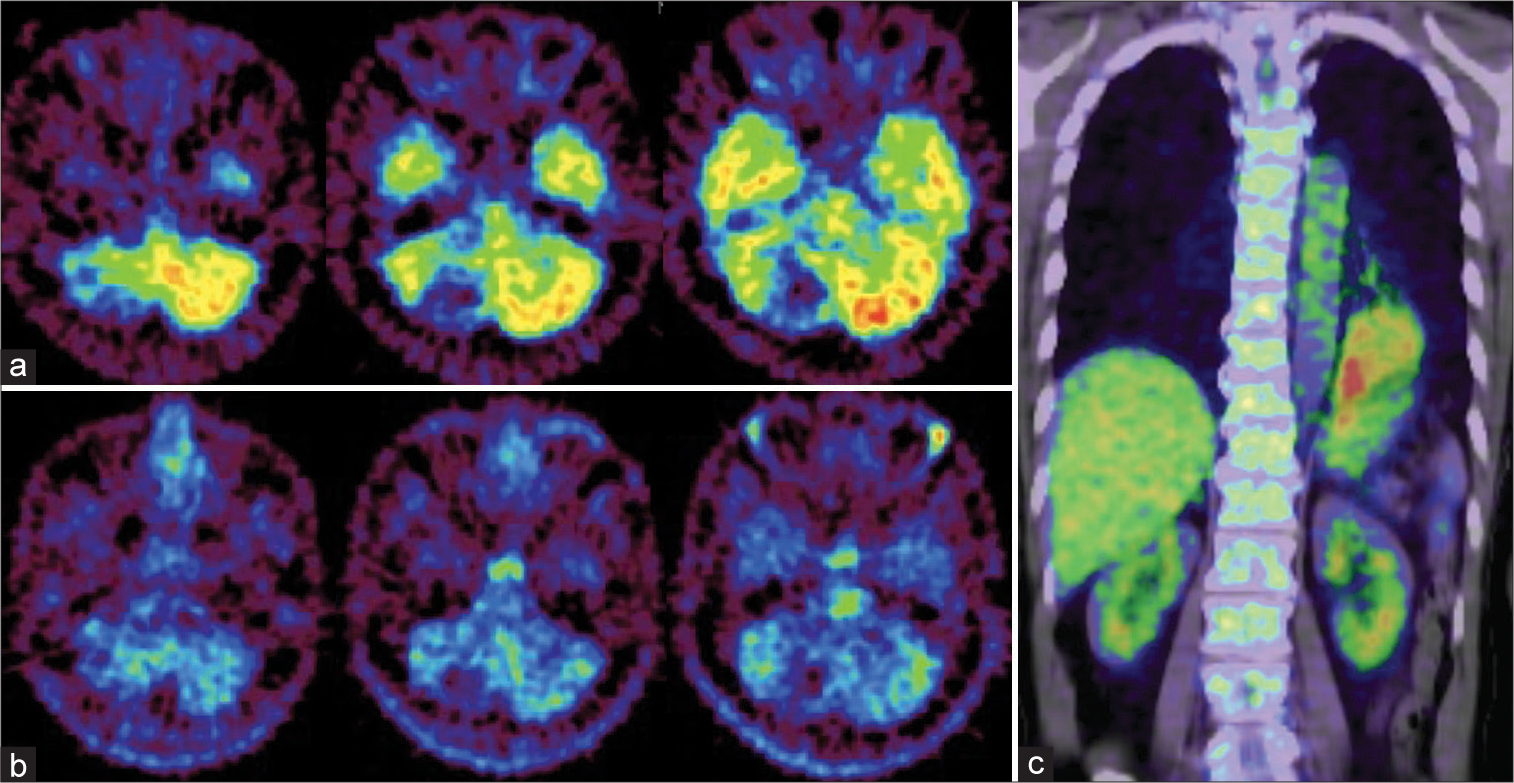
Figure 2:
Positron emission tomography (PET) of the brain and lung. (a and b) Images of brain PETs. Neither 18F-fluorodeoxyglucose (FDG)-PET (a) nor 11C-methionine-PET (b) shows significant uptake in the areas identified on gadolinium-enhanced magnetic resonance imaging [Figure 1] compared to surrounding normal brain tissue. (c) Image of lung FDG-PET. A high-uptake lesion is seen in the inferior lobe of the left lung.
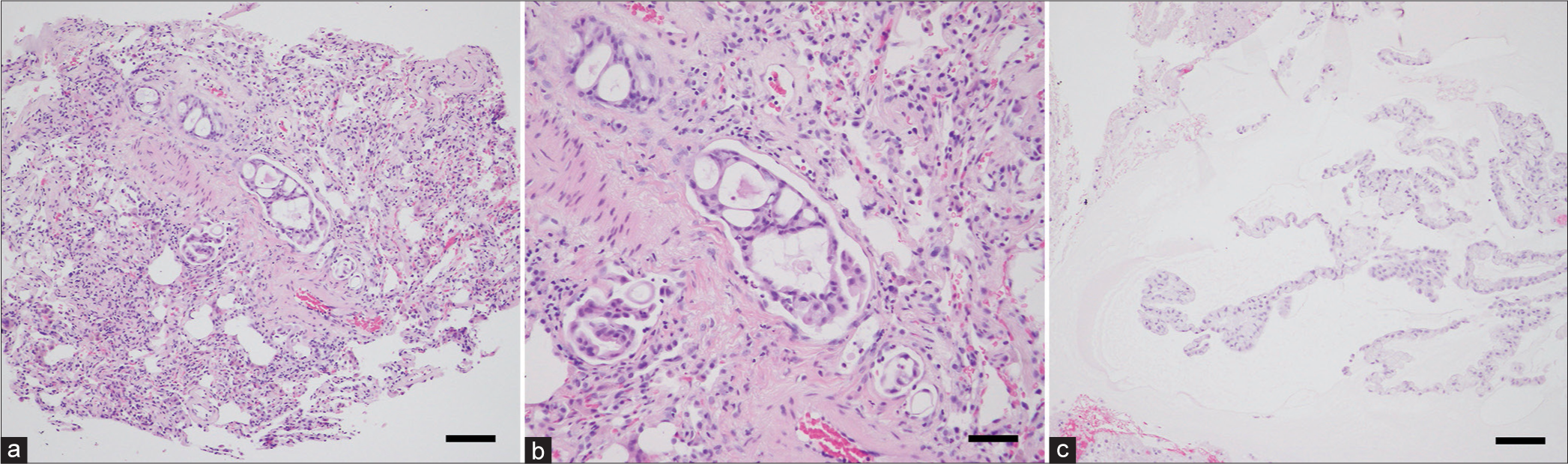
Figure 3:
Histopathological examinations of the lung and cerebellar lesions. (a and b) Acinar and cribriform patterns of tumor cells with increased chromatin and some mucin are invading the lung, showing moderately differentiated adenocarcinoma. Bar = 100 μm (a), Bar = 50 μm (b). (c) Columnar mucinous cells containing moderate cytological atypia and clear cytoplasm are forming a lepidic pattern in a mucin-rich background. The diagnosis is corresponding to the metastasis of lung adenocarcinoma. Bar = 100 μm.
Under general anesthesia, the lesions in the right cerebellar hemisphere were completely resected through a right suboccipital craniotomy in a prone position, with the lesion in the right cerebellar peduncle left untouched. The resected specimens were submitted for histopathological examination.
The postoperative course was uneventful and symptoms ameliorated. Histopathological examination revealed that the cerebellar lesions represented adenocarcinoma consistent with the lung lesion. However, more mucus-rich components were observed in the cerebellar lesions [
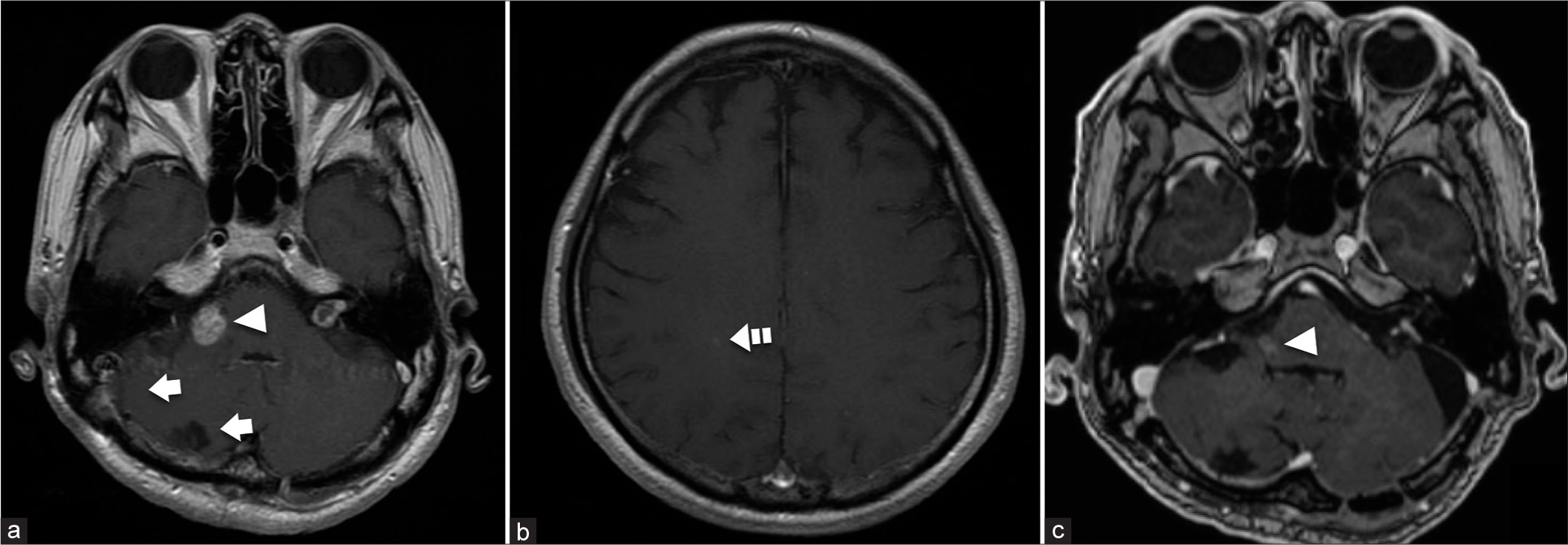
Figure 4:
Gadolinium-enhanced magnetic resonance imaging of the brain after treatment. (a and b) The two cerebellar lesions are resected, and fractionated stereotactic radiosurgery (SRS) is administered to the lesions in the right cerebellar peduncle and right parietal lobe. No evidence of recurrence is demonstrated in the right cerebellar hemisphere (arrows). The lesion in the right cerebellar peduncle shows slight shrinkage 3 months after the completion of SRS (arrowhead) (a). The lesion in the right parietal lobe is stable (dashed arrow) (b). (c) The lesion in the right cerebellar peduncle shows marked shrinkage 4 years and 5 months after the completion of SRS (arrowhead) with no recurrence of the other lesions.
DISCUSSION
FDG-PET is well known to be useful for delineating malignant tumors by assessing metabolic activity.[
The negative rate of FDG-PET has been reported as 32–39% in metastatic brain tumors detectable on CT or MRI.[
The histological class of “colloid/mucinous/lepidic” in the international multidisciplinary classification of lung adenocarcinoma[
CONCLUSION
In addition to lesion size, histopathological features of metastatic brain tumors could be associated with negative findings on MET-PET as well as FDG-PET, which can contradict findings from the primary lesion. Proper assessment of results from PET studies is important when deciding on treatment strategies for metastatic brain tumors.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
This work was supported by a Grant-in-Aid for Early-Career Scientists awarded to K.T. (no. 20K17928) from the Japan Society for the Promotion of Science (JSPS).
Conflicts of interest
There are no conflicts of interest.
References
1. Bergström M, Collins VP, Ehrin E, Ericson K, Eriksson L, Greitz T. Discrepancies in brain tumor extent as shown by computed tomography and positron emission tomography using [68Ga]EDTA, [11C]glucose, and [11C]methionine. J Comput Assist Tomogr. 1983. 7: 1062-6
2. Ericson K, Lilja A, Bergström M, Collins VP, Eriksson L, Ehrin E. Positron emission tomography with ([11C] methyl)-L-methionine, [11C]D-glucose, and [68Ga]EDTA in supratentorial tumors. J Comput Assist Tomogr. 1985. 9: 683-9
3. Griffeth LK, Rich KM, Dehdashti F, Simpson JR, Fusselman MJ, McGuire AH. Brain metastases from non-central nervous system tumors: Evaluation with PET. Radiology. 1993. 186: 37-44
4. Janus TJ, Kim EE, Tilbury R, Bruner JM, Yung WK. Use of [18F]fluorodeoxyglucose positron emission tomography in patients with primary malignant brain tumors. Ann Neurol. 1993. 33: 540-8
5. Kubota K. From tumor biology to clinical pet: A review of positron emission tomography (PET) in oncology. Ann Nucl Med. 2001. 15: 471-86
6. Lee HY, Chung JK, Jeong JM, Lee DS, Kim DG, Jung HW. Comparison of FDG-PET findings of brain metastasis from non-small-cell lung cancer and small-cell lung cancer. Ann Nucl Med. 2008. 22: 281-6
7. Lococo F, Galeone C, Formisano D, Bellafiore S, Filice A, Annunziata T. 18F-fluorodeoxyglucose positron emission tomographic scan in solid-type p-stage-I pulmonary adenocarcinomas: what can produce false-negative results?. Eur J Cardiothorac Surg. 2017. 51: 667-73
8. Matsuo M, Miwa K, Shinoda J, Kako N, Nishibori H, Sakurai K. Target definition by C11-methionine-PET for the radiotherapy of brain metastases. Int J Radiat Oncol Biol Phys. 2009. 74: 714-22
9. Mosskin M, Ericson K, Hindmarsh T, von Holst H, Collins VP, Bergström M. Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference. Acta Radiol. 1989. 30: 225-32
10. Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016. 35: 75-91
11. Rohren EM, Provenzale JM, Barboriak DP, Coleman RE. Screening for cerebral metastases with FDG PET in patients undergoing whole-body staging of non-central nervous system malignancy. Radiology. 2003. 226: 181-7
12. Singhal T, Narayanan TK, Jain V, Mukherjee J, Mantil J. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol. 2008. 10: 1-18
13. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y. International Association for the study of lung cancer/American thoracic society/European respiratory society: International multidisciplinary classification of lung adenocarcinoma: Executive summary. Proc Am Thorac Soc. 2011. 8: 381-5
14. Tyler JL, Diksic M, Villemure JG, Evans AC, Meyer E, Yamamoto YL. Metabolic and hemodynamic evaluation of gliomas using positron emission tomography. J Nucl Med. 1987. 28: 1123-33
15. Yamaguchi S, Hirata K, Okamoto M, Shimosegawa E, Hatazawa J, Hirayama R. Determination of brain tumor recurrence using (11) C-methionine positron emission tomography after radiotherapy. Cancer Sci. 2021. 112: 4246-56