- Department of Brain Sciences, Imperial College London, England, United Kingdom
- Department of Surgery and Cancer, Neuroradiology, Imperial College London, England, United Kingdom
- Department of Neurosurgery, Queens Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, England, United Kingdom
- Department of Neuro-oncology, Imperial College of London, London, England, United Kingdom
- Department of Imaging, Imperial College of London, London, England, United Kingdom
- Centre for Clinical Brain Science, University of Edinburgh, Edinburgh, Scotland, United Kingdom
Correspondence Address:
Giulio Anichini, Department of Brain Sciences, Imperial College of London, London, United Kingdom.
DOI:10.25259/SNI_369_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Giulio Anichini1, Islam Shah2, Dominic Edward Mahoney3, Neekhil Patel1, Lillie Pakzad-Shahabi4, Olga Fadeeva Da Costa5, Nelofer Syed1, Richard Perryman1, Adam Waldman6, Kevin O’Neill1. 3D ultrasound-augmented image guidance for surgery of high-grade gliomas – A quantitative analysis focused on the extent of resection. 13-Sep-2024;15:324
How to cite this URL: Giulio Anichini1, Islam Shah2, Dominic Edward Mahoney3, Neekhil Patel1, Lillie Pakzad-Shahabi4, Olga Fadeeva Da Costa5, Nelofer Syed1, Richard Perryman1, Adam Waldman6, Kevin O’Neill1. 3D ultrasound-augmented image guidance for surgery of high-grade gliomas – A quantitative analysis focused on the extent of resection. 13-Sep-2024;15:324. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13101
Abstract
Background: We have retrospectively reviewed our series of brain tumor patients operated on using 3D IntraOperative UltraSound (IOUS) to report technical advantages and areas of improvement.
Methods: Clinical and radiological data of patients with a diagnosis of high-grade glioma IV operated with and without IOUS were retrieved and analyzed.
Results: We have found 391 patients operated using IOUS coupled with neuronavigation and 257 using neuronavigation standalone. We have selected a pool of 60 patients with a diagnosis of GlioBlastoma (GB), comparing two equally sized groups operated with and without IOUS, respectively. The average extent of resection (EOR) in the IOUS group was 93%, while in the control group, it was 80%. IOUS was significantly associated with improved EOR (P
Conclusion: Intraoperative ultrasound coupled with image guidance is associated with an improved EOR and possibly an improved OS. While we are aware of several limitations related to the present analysis, these data support the routine use of IOUS as a safe and reliable technology. Larger, prospective series with updated IOUS technology are desirable to verify the accuracy of these results.
Keywords: 3D ultrasound, High-grade glioma, Intraoperative ultrasound, Neuro-oncology, Ultrasound
INTRODUCTION
The research on new intraoperative devices to improve brain tumor resection is an ever-developing field in neurosurgery. Traditional neuronavigation systems use preoperative computer tomography (CT) or magnetic resonance imaging (MRI) data to plan surgical procedures and guide surgeons to tumor margins, allowing a greater degree of precision, safer surgery, and a more complete tumor resection that correlates with clinical outcomes.[
Intra operative ultrasound (IOUS) is an appealing solution, offering a cheaper, faster, and more accessible alternative to intraoperative MRI (iMRI). However, it is still not considered the imaging modality of choice by many surgeons. In the past, IOUS has been criticized for its poor spatial resolution, and the loss of image quality to surgical artifacts (air, blood, and instruments) detracts from its usefulness in tumor resection surgery.[
The image fusion of IOUS and preoperative MRI has improved the quality of tumor resection and the clinical outcome of surgery[
The present study is a retrospective review of our experience using intraoperative 3D-IOUS. We have performed a subgroup analysis on a cohort of patients affected by high-grade gliomas grade IV (HGG-IV) who underwent IOUS-aided surgical resection and compared them with a control group operated using a standard, standalone neuronavigation system.
Aims
This study retrospectively examines data from a series of HGG-IV surgeries. We investigated whether combining 3D intraoperative ultrasound (3D IOUS) with neuronavigation improves the extent of resection (EOR) compared to standard neuronavigation alone. In addition, we sought to discern any survival benefits in terms of overall survival (OS) and progression-free survival (PFS).
MATERIALS AND METHODS
Neuronavigation systems – technical features
This study utilized the data from surgeries assisted by two primary neuronavigation systems. Ethical clearance for data usage was granted by the Health and Research Authority – Bloomsbury Research Committee, London. REC reference: 19/LO/1763, Integrated Research Application System project ID 265404.
As mentioned, two specific devices were considered for this purpose.
SonoWand©
A combined 3D-IOUS neuronavigation device, Sonowand, was used to retrieve the data for the exposure group of this study - see Lindseth et al. detailed technical specifications.[
Figure 1:
3D-IOUS intraoperative scanning. Left: Intraoperative picture showing the dedicated tracking device coupled with the probe during and IntraOperative UltraSound (IOUS) acquisition, which links the intraoperative magnetic resonance imaging scan with the IOUS images. Center and right: A case showing two IOUS acquisitions, one performed after exposing the cortical surface (center), the other at the end of the resection (right), showing the resection cavity.
Medtronic© neuronavigation
Medtronic stealth neuronavigation, a globally recognized neuronavigation system known for its accuracy and reliability, was used to retrieve the data for the control group of this study. Unlike SonoWand™, it does not incorporate IOUS, making it ideal for the purposes of our comparison. The device utilizes an infrared tracking system and offers registration through fiducial or anatomical landmarks. It also features a surface tracer option [
The process of image registration for both devices is similar, but aside from the obvious difference of the IOUS coupling or lack thereof, the SonoWand system allows for multiple intraoperative reads and acquisitions, thus providing a real-time image at any point of surgical resection and partially adjusting for imaging shift [
Figure 3:
Flow chart showing the different acquisition techniques. Aside from the obvious difference that the SonoWand© can be coupled with an IntraOperative UltraSound (IOUS) probe, it allows for multiple acquisitions at any point during tumor resection, thus providing different views even during and at the end of the resection. IOUS probes can also be used free hand, without coupling with neuronavigation.
Data collection
Population samples have been selected from the data stored on three neuronavigation devices. Collected cases were divided into two categories:
Exposure group
Patients operated using the 3D-IOUS-neuronavigation integrated system. Two identical and interchangeable 3D-IOUS-stealth coupled devices (marked 51 and 55) were used to extract data.
Control group
Patients operated using the standalone neuronavigation system (Medtronic© Neuronavigation).
Regarding the inclusion criteria, we have collected all HGG-IV cases where the surgeon was planning to achieve a gross total resection (GTR) (100%) or at least a near total resection (NTR) (>90%) of the tumor, as per the operative note record entry and excluded all cases where a partial debulking or a biopsy was intentionally performed. Cases were selected by matching the intraoperative stealth and IOUS scan details and those into the operative notes where a surgeon clearly documented that, in his/her opinion, GTR or NTR was achieved. Patients undergoing surgery without the use of any neuronavigation devices were also excluded. Cases were selected based on a homogeneous timeframe, meaning all patients included were operated during the same period. Six senior surgeons were equally involved in the treatment of patients from the two groups. Out of this group of consultants, three anonymized surgeons (C1, C2, and C3) were dedicated neuro-oncology surgeons, and most of the cases of the present series were treated under their care. All selected patients had a preoperative and an early postoperative (<48 h) volumetric MRI scan T1 weighted (T1w) with gadolinium (Gd). Patients without volumetric images were omitted. Patients undergoing postoperative CT scan as a radiological method to check for the EOR were also excluded. The selection of cases was randomly performed by a research fellow (author GA), who operated on a random selection of all patients with a diagnosis of HGG-IV and with the appropriate inclusion criteria. Specifically, once the inclusion criteria screening was performed, the cases were randomly selected from the available pool by removing all identifiable data, assigning a random number using a randomization function on the dedicated database, and subsequently extracting an equal number of cases from the exposure and control group for transfer to the software for statistical analysis (see below). All the senior surgeons were kept blind to the results.
The following clinical data have been collected from both groups: age at diagnosis, sex, comorbidities, consultant neurosurgeon responsible for the procedure, adjuvant treatment, OS, PFS, and performance status (PS). Pre and postoperative radiological data in the form of pre and postoperative volumetric MRI scans have been collected for each patient to establish tumor volume and the presence of postoperative residual. Pre and postoperative volumetric calculations on tumor volumes and postoperative residuals (when present) have been performed on the sequences mentioned above by a dedicated neuroradiology team. GTR was defined as the absence of enhancing residual on an early (<48 h) postoperative volumetric T1w with a Gd MRI scan. Blind imaging data were provided to the radiology team (authors IS, OC, and AW) so that they were not aware of which group the patient was extracted from (exposure vs. control) to limit confirmation bias. The location of the tumors in terms of depth and proximity to cortical and subcortical eloquent areas has also been considered in our analysis.
The primary outcome considered for the present study was the EOR in the two groups in terms of both volume reduction and the presence/absence of the residual. The secondary outcomes were the OS and PFS in the two groups.
Statistical analysis
Statistical analysis was performed using RStudio (version 2021.09.2) running R (version 4.1.2). A linear regression model was built to test the effects of each variable on the EOR in R. The following variables were considered: use of IOUS, sex, age at diagnosis, depth of the most superficial and deepest point of the tumor, tumor size, proximity to an eloquent area, surgeon operating, and whether the case was a recurrent HGG-IV or not. Regarding the secondary outcomes (OS and PFS), a Cox proportional hazard model was built to weigh different factors’ roles. In addition to the previously mentioned fields, the following ones were considered: peri-operative PS, percentage of volume resected, MethylGuanine MethylTransferase (MGMT) and Isocitrate DeHydrogenase (IDH) status, postoperative chemoradiotherapy (CRT), and the presence of peri-operative complications. Kaplan–Meier curves were built to compare OS and PFS for the following variables: use of IOUS (Y vs. N), peri-operative complications (Y vs. N), and postoperative treatment (no CRT, palliative CRT, or radical CRT). Plots were generated in GraphPad Prism (ver. 9.3.1). Statistical significance was determined using the Mann–Whitney U-test (box-and-whisker plots) or log-rank (Mantel-Cox) test for Kaplan–Meier curves.
RESULTS
We have retrieved 391 tumor patients operated using either of our two 3D-IOUS-stealth coupled machines (numbered 55 and 51) and 257 operated using stealth neuronavigation. The remaining patients were operated using different neuronavigation devices or without neuronavigation.
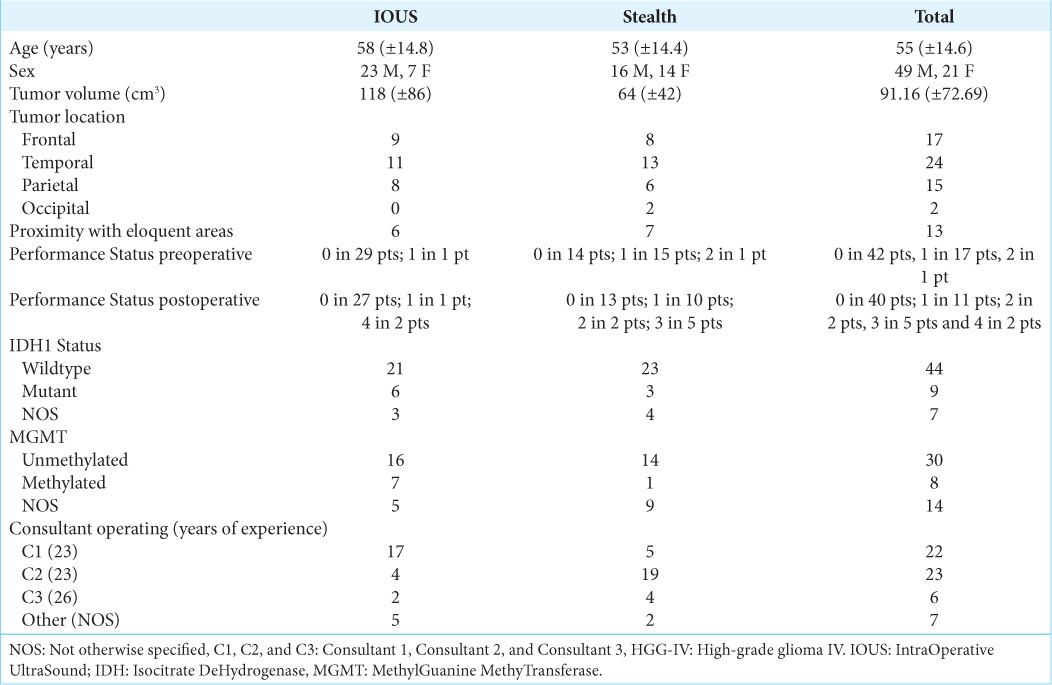
Thirty HGG-IV cases operated using IOUS-stealth coupled devices, and thirty operated using Medtronic were randomly selected from the pool of cases fitting the inclusion criteria. All cases included in the analysis had surgery between the 1st of January 2014 and the 31st of December 2017. Those cases’ clinical and radiological features are summarized in
The average tumor volume for the whole pool of cases was 91.16 cm3 (±72.69). Tumor volume was higher in the IOUS group (mean 118 cm3, ±86 cm3) compared to the control group (mean 64.14 cm3, ±42 cm3, p = 0.007) [
Figure 4:
(a) Box-and-whisker plot of preoperative volumes. Cases included in the 3D-IOUS group were found to have larger volumes compared to those included in the control group. (b) Box-and-whisker plot showing the differences in extent of resection between the group where 3D-IOUS was used, and the one where it was not (Mann–Whitney U-test was used, P < 0.0004). IOUS: Intraoperative ultrasound This correlation was found to be independent from other variables. Y = Yes. N = No; ** and *** = strength of the p value expressed in decimals (example: *** meaning p = 0.000x).
Five patients overall were operating using 5-aminolevulinic acid (5-ALA), 2 in the 3D-IOUS group and 3 in the control group. The routine use of 5-ALA in HGG-IV surgery was introduced as standard in clinical practice in the UK only in 2018. This was not part of our routine practice before that date, and given the exiguous number of patients treated using 5-ALA, this factor was not included in our final analysis.
As expected, EOR was found not to be normally distributed, with two clear peaks seen in the density distribution, separating a large population with optimal resection (n = 51, mean 92% ± 8%) and a small population with suboptimal resection (n = 9, mean 55% ± 6%). The average EOR in the IOUS group was 93% (±10%), while in the control group, it was 80% (±17%). There was a significant increase in EOR in the IOUS group compared to the control group, independent of other variables [
The IDH1 gene has been reported as wildtype in 44 cases, mutant in 9, and was not analyzed in the remaining 7 cases. The MGMT gene was unmethylated in 33 cases, methylated in 12 cases, and was not analyzed in the remaining 15 cases.
The preoperative PS was 0 in 42 patients, 1 in 17 patients, and 2 in one patient. Immediate postoperative PS was 0 in 40 patients, 1 in 11 patients, 2 in 2 patients, 3 in 5 patients, and 4 in 2 patients. Four patients experienced significant postoperative complications: Two in the form of severe postoperative cognitive and neurological impairment (PS 4), and two developed acute hydrocephalus postoperative, treated with ventriculoperitoneal shunting. One of the patients with hydrocephalus also developed aspiration pneumonia, which was successfully treated with antibiotics. We did not observe postoperative ischemic complications in either of the groups. Where observed, the worsening postoperative status was in all cases related to postoperative edema.
The average OS was 13.4 months (±8), and the average PFS was 7.4 months (±7).
The Cox proportional hazard model showed a significant advantage in OS on patients operated using the 3D-IOUS, with a hazard ratio (HR) = 0.196 and P = 0.017. As expected, administration of radical CRT was also associated with improved OS (HR = 0.199, P = 0.006). The presence of postoperative complications significantly affected survival (HR = 15.612, P = 0.002). We also observed differences in OS based on the presence of the tumor near an eloquent area (HR = 0.349, P = 0.013), the tumor volume (HR = 1.007, P = 0.047), and the surgeons performing the operations, both the “other” group (HR = 11.729, P = 0.004) and surgeon C3 (HR = 7.703, P = 0.013). However, only the peri-operative complications and application of CRT had independent effects on survival times [
Figure 5:
(a) While not independently significant, we have found a shift toward longer survival in the 3D- IOUS cohort. Perioperative complications were found to have a highly significant impact: three patients showed markedly shorter overall survival. Radical chemoradiotherapy (CRT) also significantly extends survival times, in keeping with the results of the known literature. P-values were determined by log-rank (Mantel-Cox) test. (b) Overall, progression-free survival (PFS) is poor in all patients, and we found that the differences between groups are not substantially significant. 3D-IOUS does not appear to be significantly associated with significantly improved PFS. Radical CRT promotes longer PFS, as expected. P-values determined by log-rank (Mantel-Cox) test. IOUS: IntraOperative Ultrasound, Y: Yes, N: No, OS: Overall Survival, CRT: ChemoRadioTherapy, PFS: Progression Free Survival.
DISCUSSION
Historical background and 3D IOUS development
The first ever reported application of the US in neurosurgery was on a postmortem case of a 54-year-old woman.[
An extensive systematic review and meta-analysis focused on IOUS has shown an average GTR of 77%, an 82% concordance rate between IOUS and postoperative MRI scan, and high sensitivity/specificity at the beginning of surgical resection (>90%).[
Our results
Our study delves into the utility of 3D-IOUS in HGGIV surgeries. In general, HGG-IV and anaplastic gliomas grade III presented diverse IOUS results. While anaplastic astrocytomas and oligodendrogliomas were hard to discern due to mixed boundaries with surrounding edema, necrotic and cystic areas were typically discernible. For this study, HGG-IV was chosen due to its prevalence and because there is consensus on defining the EOR by looking at the enhancing component. On IOUS, HGG-IVs mirrored MRI features: the enhancing capsule was hyper-echogenic, whereas the necrotic core was hypoechogenic. Recurrent HGG-IV and recurrent anaplastic grade III are more challenging tumors to visualize on 3D-IOUS, in our experience [
Figure 6:
(a) A right frontal high-grade glioma as seen on the SonoWand neuronavigation sequence alone (left) and with the same sequence co-registered with 3D-IOUS. Visualization of tumor boundaries is adequate, although the lesion often appears homogeneously hyperechogenic, with no clear distinction between the tumor capsule and the necrotic core. (b) A case of left frontal recurrent glioblastoma. Apart from the size of the lesion requiring multiple acquisitions and therefore causing linear artifacts, the tumor appears challenging to define on IOUS due to the relatively homogeneous hyperechogenic signal. IOUS: Intraoperative ultrasound
Figure 7:
Intraoperative picture of right posterior temporal glioblastoma before (left) and after resection (right). In this case, the necrotic core was well visualized. On the right side, note the presence of a partial shift of the brain surface and a cone of hyperechogenic material due to the deposit of blood products at the bottom of the cavity.
We have included two comparable groups of HGG-IVs operated by a heterogeneous pool of surgeons. This selection aimed at choosing a homogeneous group of patients, tumors, and treatment groups to address how much the EOR would change with or without the IOUS and whether there could be an impact on OS and PFS. The tumors included had different locations, sizes, and proximity to the surface. However, the number of tumors located near eloquent areas was similar between the exposure and the control group (6 vs. 7, respectively). Moreover, the average depths of the tumors were comparable between the two groups in terms of the most superficial and the deepest portions. On the other hand, preoperative tumor volumes were higher in the 3D-IOUS group than in the control group, which was presumably a stochastic effect. Despite the average larger tumor volume pointing toward a potentially more challenging surgical resection, the EOR was still more generous in the 3D-IOUS group, thus suggesting that IOUS makes a difference in the quality of EOR regardless of tumor size. Our results show that, when all possible factors are considered, the EOR is increased when IOUS is used. This result aligns with those from the international literature [
Limitations
The present data collection and relative analysis have been conducted to the best of our knowledge without selection biases and limiting access to nonblind data to all the authors involved in the analysis. However, we are aware that the present study still has several limitations. First, this is a retrospective review based on data previously collected. We have, therefore, excluded a great number of cases due to incomplete or missing pools of data. For example, many patients have not been scanned within 48 hours postoperative, and in many others, we found a lack of a volumetric T1 with Gd scan, either pre or postoperative. We believe that it was crucial for homogeneous data to retrieve only those cases where all the inclusion criteria were strictly followed. Still, this decision has inevitably reduced the number of cases we could include. Second, the dataset is quite heterogeneous, and some of the surgeons clearly preferred using the 3D-IOUS to start with because of the integration between neuronavigation and the US. This is the main reason why we have included the operating surgeon as a possible confounding variable in the statistical analysis. Still, given the small pools of cases analyzed, this factor might have impacted more than shown. Third, the small sample included in the analysis did not allow for a more precise stratification of the data, meaning that tumors similar in locations and size could not be precisely compared. In our analysis, we have partially tried to solve this problem by accounting for tumor location in terms of eloquence proximity, preoperative size, and depth. However, ideally, it would have been more adequate to compare outcomes from pools of tumors in similar locations. It is also worth noting that eloquent area proximity is an outdated concept [
CONCLUSION
The present statistical analysis on HGG-IV cases shows an advantage in EOR using IOUS compared to the cases where IOUS is not used. This is in keeping with the more recent results from the international literature and highlights the possibility that an improved EOR is achieved when IOUS is deployed by an experienced team. Our analysis also suggests a potential advantage in OS in those cases where IOUS is used, and this holds even when considering other factors known to affect survival, such as complications, administration of postoperative radical CRT, PS, and tumor size. The OS finding needs further studies to be confirmed, as PFS seems to be unaffected by IOUS. Finally, IOUS and tumor resections remain heavily operator-dependent, suggesting that the results of the present analysis might have been affected by the limitations mentioned above. A prospective analysis of a broader pool of patients, stratified according to a more precise tumor location, surgical technique, and preoperative planning, is desirable to verify these findings.
Ethical approval
The research/study approved by the Institutional Review Board at Research Authority – Bloomsbury Research Committee, number 19/LO/1763, dated October 17, 2019.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
The present study did not receive specific fundings. Mr Giulio Anichini, Mr Kevin O’Neill, Dr Richard Perryman and Dr Nelofer Syed are supported by the charities Brain Tumour Research (BTR) and Brain Tumour Research Campaign (BTRC).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary Material
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Arlt F, Chalopin C, Muns A, Meixensberger J, Lindner D. Intraoperative 3D contrast-enhanced ultrasound (CEUS): A prospective study of 50 patients with brain tumours. Acta Neurochir (Wien). 2016. 158: 685-94
2. Bonosi L, Marrone S, Benigno UE, Buscemi F, Musso S, Porzio M. Maximal safe resection in glioblastoma surgery: A systematic review of advanced intraoperative image-guided techniques. Brain Sci. 2023. 13: 216
3. Carpenter DJ, Leng J, Arshad M, Giles W, Kirkpatrick JP, Floyd SR. Intracranial and extracranial progression and their correlation with overall survival after stereotactic radiosurgery in a multi-institutional cohort with brain metastases. JAMA Netw Open. 2023. 6: e2310117
4. Cepeda S, García-García S, Arrese I, Velasco-Casares M, Sarabia R. Relationship between the overall survival in glioblastomas and the radiomic features of intraoperative ultrasound: A feasibility study. J Ultrasound. 2022. 25: 121-8
5. Chowdhury S, Mainwaring P, Zhang L, Mundle S, Pollozi E, Gray A. Systematic review and meta-analysis of correlation of progression-free survival-2 and overall survival in solid tumors. Front Oncol. 2020. 10: 1349
6. Dixon L, Lim A, Grech-Sollars M, Nandi D, Camp S. Intraoperative ultrasound in brain tumor surgery: A review and implementation guide. Neurosurg Rev. 2022. 45: 2503-15
7. Dohrmann GJ, Rubin JM. History of intraoperative ultrasound in neurosurgery. Neurosurg Clin N Am. 2001. 12: 155-66
8. Duffau H. A two-level model of interindividual anatomo-functional variability of the brain and its implications for neurosurgery. Cortex. 2017. 86: 303-13
9. Enchev Y. Neuronavigation: Geneology, reality, and prospects. Neurosurg Focus. 2009. 27: E11
10. French LA, Wild JJ, Neal D. Detection of cerebral tumors by ultrasonic pulses; pilot studies on postmortem material. Cancer. 1950. 3: 705-8
11. Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: Prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE. 3. Lancet Oncol. 2008. 9: 29-38
12. Gronningsaeter A, Kleven A, Ommedal S, Aarseth TE, Lie T, Lindseth F. SonoWand, an ultrasound-based neuronavigation system. Neurosurgery. 2000. 47: 1373-9 discussion 1379-80
13. Gumprecht HK, Widenka DC, Lumenta CB. BrainLab vectorvision neuronavigation system: Technology and clinical experiences in 131 cases. Neurosurgery. 1999. 44: 97-104 discussion 104-5
14. Hata N, Dohi T, Iseki H, Takakura K. Development of a frameless and armless stereotactic neuronavigation system with ultrasonographic registration. Neurosurgery. 1997. 41: 608-13 discussion 613
15. Incekara F, Smits M, Dirven L, Bos EM, Balvers RK, Haitsma IK. Intraoperative B-mode ultrasound guided surgery and the extent of glioblastoma resection: A randomized controlled trial. Front Oncol. 2021. 11: 649797
16. Ji S, Roberts DW, Hartov A, Paulsen KD. Intraoperative patient registration using volumetric true 3D ultrasound without fiducials. Med Phys. 2012. 39: 7540-52
17. Lindner D, Trantakis C, Renner C, Arnold S, Schmitgen A, Schneider J. Application of intraoperative 3D ultrasound during navigated tumor resection. Minim Invasive Neurosurg. 2006. 49: 197-202
18. Lindseth F, Kaspersen JH, Ommedal S, Lango T, Bang J, Hokland J. Multimodal image fusion in ultrasound-based neuronavigation: improving overview and interpretation by integrating preoperative MRI with intraoperative 3D ultrasound. Comput Aided Surg. 2003. 8: 49-69
19. Mahboob S, McPhillips R, Qiu Z, Jiang Y, Meggs C, Schiavone G. Intraoperative ultrasound-guided resection of gliomas: A meta-analysis and review of the literature. World Neurosurg. 2016. 92: 255-63
20. Mauer M, Stupp R, Taphoorn M, Coens C, Osoba D, Marosi C. The prognostic value of health-related quality-of-life data in predicting survival in glioblastoma cancer patients: Results from an international randomised phase III EORTC Brain Tumour and Radiation Oncology Groups, and NCIC Clinical Trials Group study. Br J Cancer. 2007. 97: 302-7
21. Moiraghi A, Prada F, Delaidelli A, Guatta R, May A, Bartoli A. Navigated intraoperative 2-dimensional ultrasound in high-grade glioma surgery: Impact on extent of resection and patient outcome. Oper Neurosurg (Hagerstown). 2020. 18: 363-73
22. Neidert MC, Hostettler IC, Burkhardt JK, Mohme M, Held U, Kofmehl R. The influence of intraoperative resection control modalities on survival following gross total resection of glioblastoma. Neurosurg Rev. 2016. 39: 401-9
23. Ohue S, Kumon Y, Nagato S, Kohno S, Harada H, Nakagawa K. Evaluation of intraoperative brain shift using an ultrasound-linked navigation system for brain tumor surgery. Neurol Med Chir (Tokyo). 2010. 50: 291-300
24. Plaha P, Camp S, Cook J, McCulloch P, Voets N, Ma R. FUTURE-GB: Functional and ultrasound-guided resection of glioblastoma-a two-stage randomised control trial. BMJ Open. 2022. 12: e064823
25. Prada F, Del Bene M, Mattei L, Lodigiani L, DeBeni S, Kolev V. Preoperative magnetic resonance and intraoperative ultrasound fusion imaging for real-time neuronavigation in brain tumor surgery. Ultraschall Med. 2015. 36: 174-86
26. Rasmussen IA, Lindseth F, Rygh OM, Berntsen EM, Selbekk T, Xu J. Functional neuronavigation combined with intra-operative 3D ultrasound: initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data. Acta Neurochir (Wien). 2007. 149: 365-78
27. Riva M, Hennersperger C, Milletari F, Katouzian A, Pessina F, Gutierrez-Becker B. 3D intra-operative ultrasound and MR image guidance: Pursuing an ultrasound-based management of brainshift to enhance neuronavigation. Int J Comput Assist Radiol Surg. 2017. 12: 1711-25
28. Roder C, Stummer W, Coburger J, Scherer M, Haas P, von der Brelie C. Intraoperative MRI-guided resection is not superior to 5-aminolevulinic acid guidance in newly diagnosed glioblastoma: A prospective controlled multicenter clinical trial. J Clin Oncol. 2023. 41: 5512-23
29. Saether CA, Torsteinsen M, Torp SH, Sundstrom S, Unsgard G, Solheim O. Did survival improve after the implementation of intraoperative neuronavigation and 3D ultrasound in glioblastoma surgery? A retrospective analysis of 192 primary operations. J Neurol Surg A Cent Eur Neurosurg. 2012. 73: 73-8
30. Unsgaard G, Ommedal S, Muller T, Gronningsaeter A, Nagelhus Hernes TA. Neuronavigation by intraoperative three-dimensional ultrasound: Initial experience during brain tumor resection. Neurosurgery. 2002. 50: 804-12 discussion 812
31. Unsgaard G, Selbekk T, Brostrup Muller T, Ommedal S, Torp SH, Myhr G. Ability of navigated 3D ultrasound to delineate gliomas and metastases--comparison of image interpretations with histopathology. Acta Neurochir (Wien). 2005. 147: 1259-69 discussion 1269
32. Wadley J, Dorward N, Kitchen N, Thomas D. Pre-operative planning and intra-operative guidance in modern neurosurgery: A review of 300 cases. Ann R Coll Surg Engl. 1999. 81: 217-25
33. Wang J, Liu X, Ba YM, Yang YL, Gao GD, Wang L. Effect of sonographically guided cerebral glioma surgery on survival time. J Ultrasound Med. 2012. 31: 757-62
34. Wirtz CR, Albert FK, Schwaderer M, Heuer C, Staubert A, Tronnier VM. The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res. 2000. 22: 354-60
35. Zhao P, Zhao Y, Zhang W, Zhang MZ, Zhao JZ. Ultrasound-guided minimally invasive neurosurgery in treatment of cranial tumors: clinical study. Zhonghua Yi Xue Za Zhi. 2006. 86: 1600-3