- Department of Neurosurgery, Sasebo City General Hospital, Sasebo City, Nagasaki, Japan
- Department of Pathology, Sasebo City General Hospital, Sasebo City, Nagasaki, Japan.
Correspondence Address:
Michiharu Yoshida, Department of Neurosurgery, Sasebo City General Hospital, Sasebo City, Nagasaki, Japan.
DOI:10.25259/SNI_204_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michiharu Yoshida1, Susumu Yamaguchi1, Keisuke Iwasaki2, Mitsuto Iwanaga1. 5-aminolevulinic acid-guided endoscopic biopsy with violet light-emitting diode flashlight in malignant glioma: Technical note. 10-Nov-2023;14:397
How to cite this URL: Michiharu Yoshida1, Susumu Yamaguchi1, Keisuke Iwasaki2, Mitsuto Iwanaga1. 5-aminolevulinic acid-guided endoscopic biopsy with violet light-emitting diode flashlight in malignant glioma: Technical note. 10-Nov-2023;14:397. Available from: https://surgicalneurologyint.com/surgicalint-articles/12629/
Abstract
Background: 5-aminolevulinic acid (5-ALA) photodynamic diagnosis (PDD) has enabled better identification of malignant tumor cells and real-time intraoperative guidance. Here, we report a reasonable procedure for 5-ALA-guided endoscopic biopsy with a violet light-emitting diode (LED) flashlight for deep-seated malignant gliomas.
Methods: A 63-year-old man presented with a headache and left upper homonymous quadrantanopia. Imaging studies showed atypical lesions with non-significant and partial contrast enhancement in the right deep temporo-occipital lobe. An endoscopic biopsy was performed under the guidance of 5-ALA PDD with a violet LED flashlight.
Results: The tumor tissues, which were difficult to distinguish from normal brain parenchyma under white light, were positive for 5-ALA fluorescence. The histopathological diagnosis was astrocytoma (the World Health Organization grade 3). The patient underwent adjuvant chemoradiation therapy. Headache and anopia improved, and no recurrence was observed at 12 months follow-up.
Conclusion: This technique of neuroendoscopic biopsy guided by 5-ALA PDD fluorescence with a violet LED flashlight may allow a safe and accurate diagnosis of deep-seated malignant gliomas.
Keywords: 5-aminolevulinic acid, Endoscopic biopsy, Light-emitting diode flashlight, Malignant glioma
INTRODUCTION
The prognosis of malignant gliomas remains poor compared to other malignant tumors in humans.[
In recent years, surgery combining neuroendoscopy and 5-ALA PDD has been used to remove and biopsy deep-seated lesions.[
MATERIALS AND METHODS
A 63-year-old right-handed man presented with a headache and left upper homonymous quadrantanopia. Magnetic resonance imaging (MRI) revealed a soft-tissue component showing hyperintensity on fluid-attenuated invasion recovery (FLAIR) images of the right deep temporo-occipital lobe. The right medial occipitotemporal gyrus was slightly enhanced along the sulcus with contrast medium on T1-weighted sequences. Magnetic resonance spectroscopy revealed elevated choline and lactate peaks and decreased creatine and N-acetyl aspartate peaks. Given these MRI findings and the patient’s medical history, an initial diagnosis of high-grade glioma was made. We considered two alternative approaches for diagnostic biopsy: endoscopic transcortical approach and needle biopsy under neuronavigation and 5-ALA PDD guidance. The endoscopic approach was preferred to decrease the risk of non-diagnostic biopsy and postoperative bleeding.
Operation
The patient received a 20-mg/kg oral dose of 5-ALA dissolved in water three h before surgery. In the supine position under general anesthesia, the head was fixed in Mayfield clamps with over 60° of rotation in the contralateral tumor-side location. A small craniotomy was performed in the right temporal region, and a corticotomy was performed in the non-eloquent cortex of the right middle temporal gyrus. After the direction of approach to the lesion was determined with a neuronavigational system (Brainlab AG, Munich, Germany), we inserted the neuronavigational probe along the inner cylinder of a transparent sheath with a diameter of 10 mm (NeuroPort; Olympus, Tokyo, Japan) using the slight contrast enhancement (CE) on MRI as a target; subsequently, the probe and inner cylinder were removed, and a 4-mm 0° rigid neuroendoscope (EndoArm; Olympus, Tokyo, Japan) was placed along the outer sheath. While the main operator watched the tumor tissue bearing a resemblance to normal parenchyma on the monitor and held the sheath and neuroendoscope, an assistant operator removed the white light attachment and replaced it with a violet LED flashlight [
RESULTS
Pathological findings
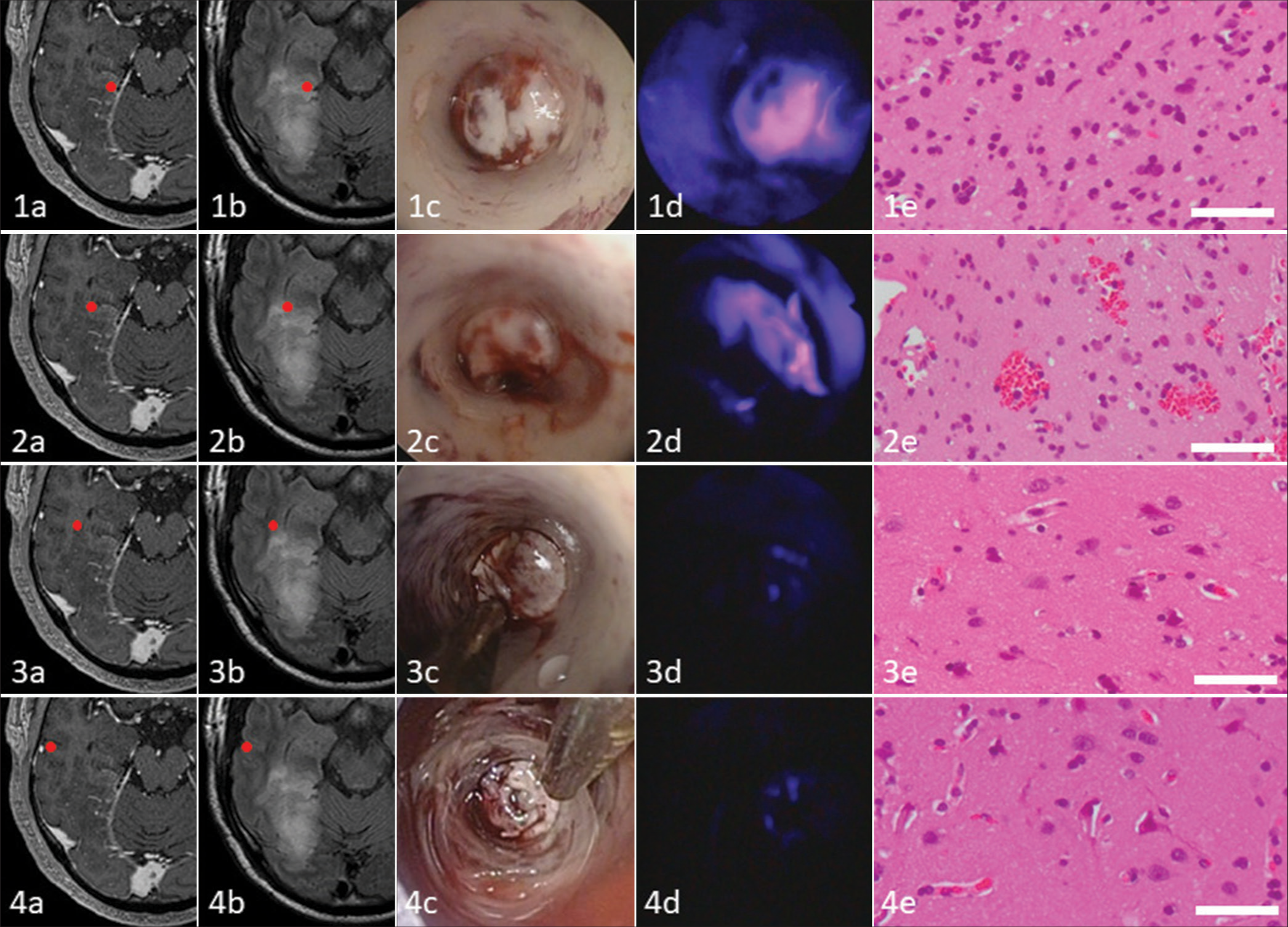
Tumor cells showed two intraoperative 5-ALA PDD fluorescence patterns [
Figure 2:
Comparison of indicated intratumoral areas in malignant glioma: Contrast enhancement (CE) on magnetic resonance imaging, fluid-attenuated invasion recovery (FLAIR) image, conventional white light endoscopy, 5-aminolevulinic acid photodynamic diagnosis fluorescence with violet light-emitting diode flashlight, and histopathology. The tissue from inside the (1a) slight CE and (1b) hyperintense on FLAIR depicts (1c) no obvious endoscopic abnormalities, (1d) strong fluorescence, and (1e) corresponding histopathology demonstrates infiltrating anaplastic tumor cells of an astrocytoma, world health organization grade 3. The tissue from inside the (2a) non-CE and (2b) hyperintense on FLAIR shows (2c) no obvious endoscopic abnormalities, reveals (2d) vague fluorescence, and (2e) histopathologically corresponds to infiltrating anaplastic tumor cells of an astrocytoma, grade 3. The tissue from the (3a) non-CE and (3b) rim of hyperintense on FLAIR shows (3c) no obvious endoscopic abnormalities, is (3d) negative for fluorescence, and (3e) histopathologically reveals no definite infiltrating tumor cells. The subcortical tissue from the (4a) non-CE and (4b) isointense on FLAIR shows (4c) no obvious endoscopic abnormalities, is (4d) negative for fluorescence, and (4e) histopathologically reveals no definite infiltrating tumor cells. Bar = 50 µm.
Postoperative course
Because the pathological diagnosis of astrocytoma was grade 3, additional adjuvant therapy was required. The patient underwent concomitant chemoradiotherapy (temozolomide, 60 Gy) and adjuvant temozolomide chemotherapy. Headache and anopia improved, and no tumor recurrence was noted at 12 months follow-up.
DISCUSSION
We report the novel assistance of a violet LED flashlight during a 5-ALA-guided endoscopic biopsy in a patient with deep-seated malignant glioma.
Although stereotactic surgery is often performed for brain tumor biopsy, blind procedures are associated with a risk of non-diagnostic samples and hemorrhagic complications.[
5-ALA is a precursor molecule of PpIX in the heme biosynthetic pathway in the mitochondria and cytoplasm.[
Our technique may offer several advantages over plain tumor biopsy without 5-ALA, including improved diagnostic accuracy and reduced normal brain damage. Contrastingly, our study has some limitations. While using an LED flashlight in our technique was simple and cost-effective, the output power of the light source was relatively low, and the observation was conducted without a filter system for rebound rays, which increased the risk of pseudo-negative and pseudo-positive results, respectively. There was a necessity for dark room observation. Otherwise, our application would be improved by wearing filter glasses or putting an ultrathin filter in front of the usual camera for reflected red light. Furthermore, this procedure requires a combination of two synchronized operators. Nevertheless, we believe that 5-ALA-guided neuroendoscopic visualization with a violet LED flashlight could enhance our surgical experience in deep-seated brain tumor surgery.
CONCLUSION
Neuroendoscopic biopsy of deep-seated malignant gliomas under 5-ALA PDD guidance with a violet LED flashlight allowed for an effective and safe diagnosis of the lesion. Although further research is needed to establish 5-ALA-guided neuroendoscopic surgery with LED flashlights, we believe this technique can be safely and accurately applied to deep brain tumors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Choo J, Takeuchi K, Nagata Y, Ohka F, Kishida Y, Watanabe T. Neuroendoscopic cylinder surgery and 5-aminolevulinic acid photodynamic diagnosis of deep-seated intracranial lesions. World Neurosurg. 2018. 116: e35-41
2. Dammers R, Haitsma IK, Schouten JW, Kros JM, Avezaat CJ, Vincent AJ. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir (Wien). 2008. 150: 23-9
3. Devalckeneer A, Aboukaïs R, Bourgeois P, Reyns N, Lejeune JP. Supraorbital transciliary approach as primary route to fronto-basal high grade glioma resection with 5-aminolevulinic acid use: Technical note: Technical note. Neurochirurgie. 2023. 69: 101387
4. Evers G, Kamp M, Warneke N, Berdel W, Sabel M, Stummer W. 5-Aminolaevulinic acid-induced fluorescence in primary central nervous system lymphoma. World Neurosurg. 2017. 98: 375-80
5. Ferraro N, Barbarite E, Albert TR, Berchmans E, Shah AH, Bregy A. The role of 5-aminolevulinic acid in brain tumor surgery: A systematic review. Neurosurg Rev. 2016. 39: 545-55
6. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014. 370: 699-708
7. Harrisson SE, Shooman D, Grundy PL. A prospective study of the safety and efficacy of frameless, pinless electromagnetic image-guided biopsy of cerebral lesions. Neurosurgery. 2012. 70: 29-33
8. Ishikawa T, Takahashi K, Ikeda N, Kajimoto Y, Hagiya Y, Ogura S. Transporter-mediated drug interaction strategy for 5-aminolevulinic acid (ALA)-based photodynamic diagnosis of malignant brain tumor: Molecular design of ABCG2 inhibitors. Pharmaceutics. 2011. 3: 615-35
9. Ishizuka M, Abe F, Sano Y, Takahashi K, Inoue K, Nakajima M. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int Immunopharmacol. 2011. 11: 358-65
10. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
11. Lu Y, Yeung C, Radmanesh A, Wiemann R, Black PM, Golby AJ. Comparative effectiveness of frame-based, frameless, and intraoperative magnetic resonance imaging-guided brain biopsy techniques. World Neurosurg. 2015. 83: 261-8
12. Mori R, Akasaki Y, Fukasawa N, Kawamura D, Karagiozov K, Murayama Y. Fully-endoscopic resection of deep-seated pilocytic astrocytoma under 5-aminolevulinic acid fluorescence guidance: A technical note. Turk Neurosurg. 2022. 32: 872-6
13. Rapp M, Kamp M, Steiger HJ, Sabel M. Endoscopic-assisted visualization of 5-aminolevulinic acid-induced fluorescence in malignant glioma surgery: A technical note. World Neurosurg. 2014. 82: e277-9
14. Rossi M, Gay L, Ambrogi F, Conti Nibali M, Sciortino T, Puglisi G. Association of supratotal resection with progression-free survival, malignant transformation, and overall survival in lower-grade gliomas. Neuro Oncol. 2021. 23: 812-26
15. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
16. Strickland BA, Wedemeyer M, Ruzevick J, Micko A, Shahrestani S, Daneshmand S. 5-Aminolevulinic acid-enhanced fluorescence-guided treatment of high-grade glioma using angled endoscopic blue light visualization: Technical case series with preliminary follow-up. J Neurosurg. 2022. 137: 1378-86
17. Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998. 42: 518-25
18. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
19. Takeda J, Nonaka M, Li Y, Isozaki H, Kamei T, Hashiba T. 5-Aminolevulinic acid fluorescence-guided endoscopic surgery for intraventricular tumors. Surg Neurol Int. 2022. 13: 302
20. Tanei T, Nakahara N, Takebayashi S, Hirano M, Nagatani T, Nishihata T. Endoscopic biopsy for lesions located in the parenchyma of the brain: Preoperative planning based on stereotactic methods. Technical note. Neurol Med Chir (Tokyo). 2012. 52: 617-21
21. Widhalm G, Wolfsberger S, Minchev G, Woehrer A, Krssak M, Czech T. 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with non-significant contrast enhancement. Cancer. 2010. 116: 1545-52