- Department of Paediatric Neurosurgery, Nelson Mandela Childrens Hospital, Parktown, Johanessburg, South Africa.
DOI:10.25259/SNI_246_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jason Labuschagne. 5-aminolevulinic acid-guided surgery for focal pediatric brainstem gliomas: A preliminary study. 08-Oct-2020;11:334
How to cite this URL: Jason Labuschagne. 5-aminolevulinic acid-guided surgery for focal pediatric brainstem gliomas: A preliminary study. 08-Oct-2020;11:334. Available from: https://surgicalneurologyint.com/surgicalint-articles/10321/
Abstract
Background: There is a growing body of literature supporting the use of 5-aminolevulinic acid (5-ALA) in the pediatric population, however, its use is still considered “off label” in this setting. In this retrospective study, we report our experience using 5-ALA in pediatric patients with focal brainstem gliomas (BSGs).
Methods: Patients younger than 16 years presenting with a newly diagnosed BSG that was focal in nature were considered suitable for treatment with 5-ALA-assisted surgery. Exclusion criteria included MRI features suggestive of a diffuse intrinsic pontine glioma. A single dose of 5-ALA was administered preoperatively. Intraoperative fluorescence was recorded as “solid,” “vague,” or “none.” The effectiveness of the fluorescence was graded as “helpful” or “unhelpful.”
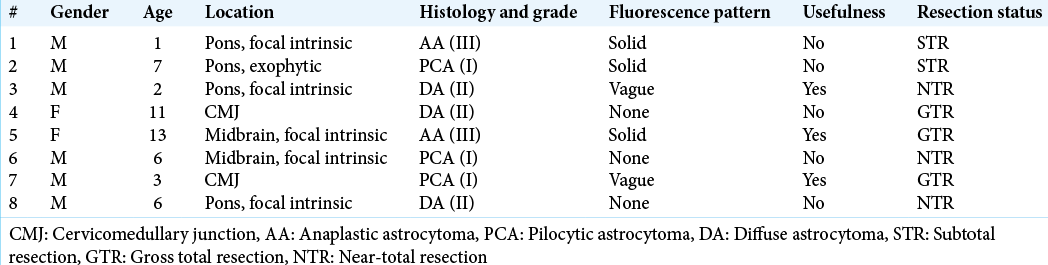
Results: Eight patients underwent 5-ALA-assisted surgery. There were four tumors located in the pons, two midbrain tumors, and two cervicomedullary tumors. Histological analysis demonstrated three diffuse astrocytomas, three pilocytic astrocytomas, and two anaplastic astrocytomas. Solid fluorescence was found in three of the eight cases, vague fluorescence was found in two cases, and no fluorescence was found in three cases. Fluorescence was useful in 3 (37%) cases. No patients experienced any complications attributable to the administration of the 5-ALA.
Conclusion: With a total fluorescence rate of 62.5% but a subjectively assessed “usefulness” rate of only 37.5%, the role of 5-ALA in BSG surgery is limited. Given the toxicological safety, however, of the agent, caution is perhaps needed before dismissing the use of 5-ALA entirely.
Keywords: 5-aminolevulinic acid, Diffuse intrinsic pontine glioma, Focal brainstem tumors, Pediatric brain tumor
INTRODUCTION
Brainstem gliomas (BSGs) account for approximately 10–20% of brain tumors in children.[
In this retrospective study, we report our experience using 5-ALA in pediatric patients with focal BSGs. The aim of the report is to analyze the safety of its use, evaluate the intraoperative fluorescence rate and the “usefulness” of 5-ALA in this specific group of patients.
METHODS
Patient selection
Patients younger than 16 years presenting with a newly diagnosed, untreated BSG that was focal in nature were considered suitable for treatment with 5-ALA-assisted surgery. Exclusion criteria included MRI features suggestive of a diffuse intrinsic pontine glioma (DIPG), preexisting hepatic or renal disease, abnormal renal or hepatic function, any known cutaneous hypersensitivity, or a first degree relative with porphyria. Parents or guardians, after being informed about the potential benefit and risks derived from the existing adult data, were offered the treatment as an off-label use. Following an explanation regarding the lack of safety and efficacy data from clinical trials in the pediatric population and the character of an individual treatment attempt, written informed consent was obtained on behalf of the children from their parents or guardians. The Human Research Ethics Committee of the medical faculty of the University of the Witwatersrand approved the scientific analyses of these cases.
Operative protocol
Patients were treated according to the previously reported adult protocol of Stummer et al.[
Clinical and radiological assessment
Patients were examined clinically during their postoperative course to assess for new neurological deficits or surgical complications. Patients were clinically assessed for signs of adverse drug reactions. The Lansky Performance Scale (LPS)[
Statistical analysis
As the number of patients was small, the data presentation is mostly descriptive. Averages were expressed as means. Relationships between categorical variables were investigated by means of Fisher’s exact test. All statistics were performed using SSPS Version 25.0 for Windows (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
RESULTS
Demographics
Among the patients, six were male (75%) and 2 were female (25%). The mean age was 6.1 years old (range 1–13 years). There were four tumors located in the pons, two midbrain tumors, and two cervicomedullary tumors.
Histology and fluorescence patterns
Histological analysis demonstrated three diffuse astrocytomas (WHO Grade II), three pilocytic astrocytomas (PCAs) (WHO Grade I), and two anaplastic astrocytomas (WHO Grade III). Solid fluorescence was found in three of the eight cases, vague fluorescence was found in two cases, and no florescence was found in three cases. The sample size is too small to make meaningful commentary regarding correlation of fluorescence to tumor histology or the WHO Grade, but 2 of the 3 (66%) cases of solid fluorescence were anaplastic astrocytomas (WHO Grade III). In two of the three cases in which there was solid, and hence potentially useful fluorescence, the surgical resection was halted due to a significant change in neurophysiological parameters occurring with extensive tumor fluorescence still visible in the surgical bed. As the presence of fluorescence did not alter the surgery in these cases, we, by definition, needed to grade them as “nonuseful.” The rate of “useful” fluorescence was thus reduced to 3 (37%) cases. Strong fluorescence or the determination of “usefulness” by the surgeon did not correlate with resection rate. [
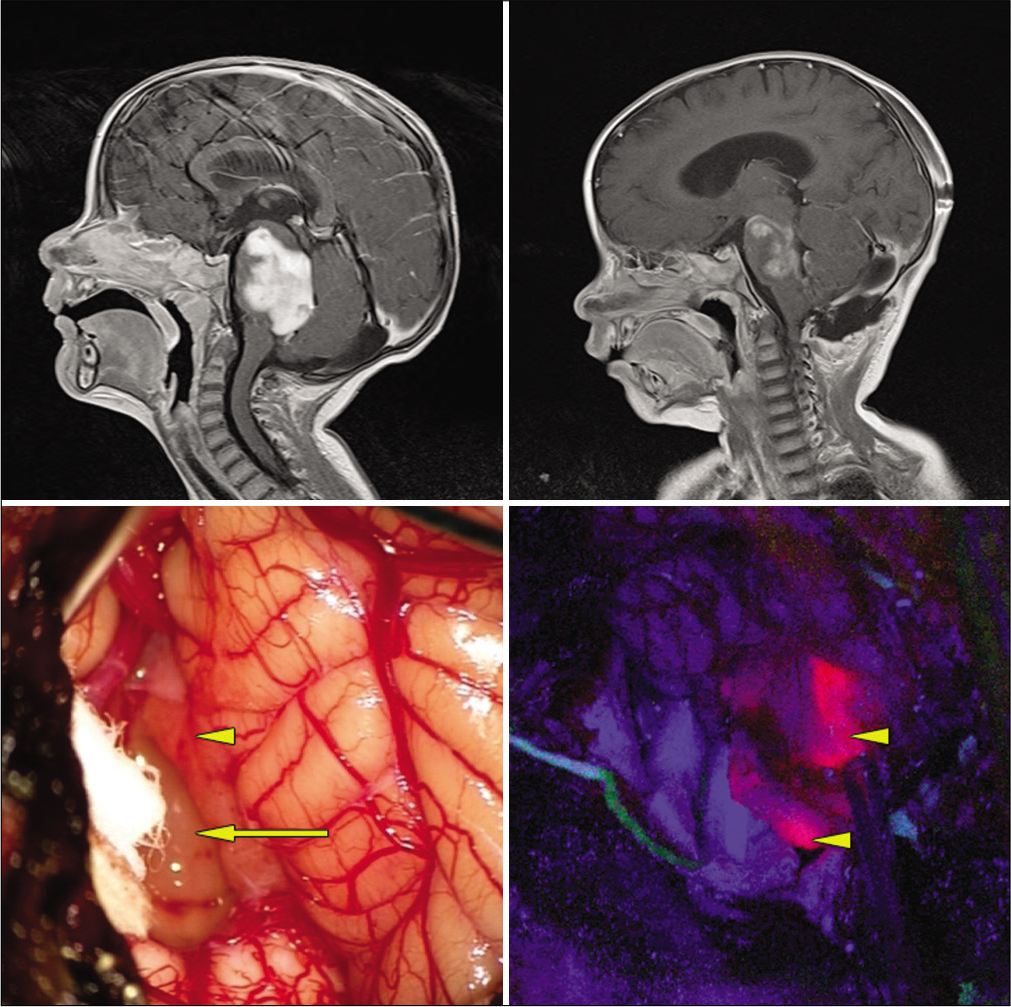
Figure 1:
Exophytic pontine pilocytic astrocytoma. Top left: preoperative, postgadolinium-enhanced sagittal MRI. Top right: postoperative gadolinium-enhanced MRI revealing subtotal resection. Bottom left: intraoperative white light microscopic view. Arrow: obvious tumor under white light microscopy. Arrowhead: normal appearing tissue under white light microscopy. Bottom right: arrow heads: blue light mode reveals solid fluorescence of both obvious tumor and normal appearing area under white light mode.
Adverse events, neurological sequelae, and outcome
No patients experienced any complications directly attributable to the administration of the 5-ALA. We achieved GTR in three cases, NTR in three cases, and STR in two cases. Five patients experienced postoperative complications, including one case of postoperative hydrocephalus requiring a permanent ventriculoperitoneal shunt, two cases of worsening ataxia, and two cases of diplopia. With rehabilitation, there were no permanent neurological deficits at 3 months follow-up in any of the patients. There were no significant differences between the presurgical and direct postsurgical (P = 0.12) or 3 months follow-up (P = 0.55) LPS results.
DISCUSSION
BSGs account for 25% of posterior fossa tumors and 10–20% of all CNS tumors in children.[
In our unit, we follow the treatment algorithm outlined by Pincus et al.[
Although there are no sanctioned radiological guidelines for the diagnosis of a DIPG,[
While there are proponents of surgery for diffuse BSGs,[
The role of 5-ALA assistance in high-grade glioma surgery is well established with increased GTR resection rates and 6-month progression-free survival rates benefitting HGG patients receiving 5-ALA.[
The histopathology of DIPG represents a spectrum with grade not predictive of survival.[
Potentially of greater significance than histological diagnosis, tumor grade, or markers of proliferation[
One of the limitations of 5-ALA-assisted surgery of lesions located in the brainstem is the impaired reliability of fluorescence in this area. Reliable fluorescence requires a sufficient exposure to fluorescent light at a perpendicular perspective to the operating microscope.[
In HGG surgery, the 5-ALA fluorescence pattern is not homogenous throughout the tumor tissue. A three-tier grading system described by Stummer et al.[
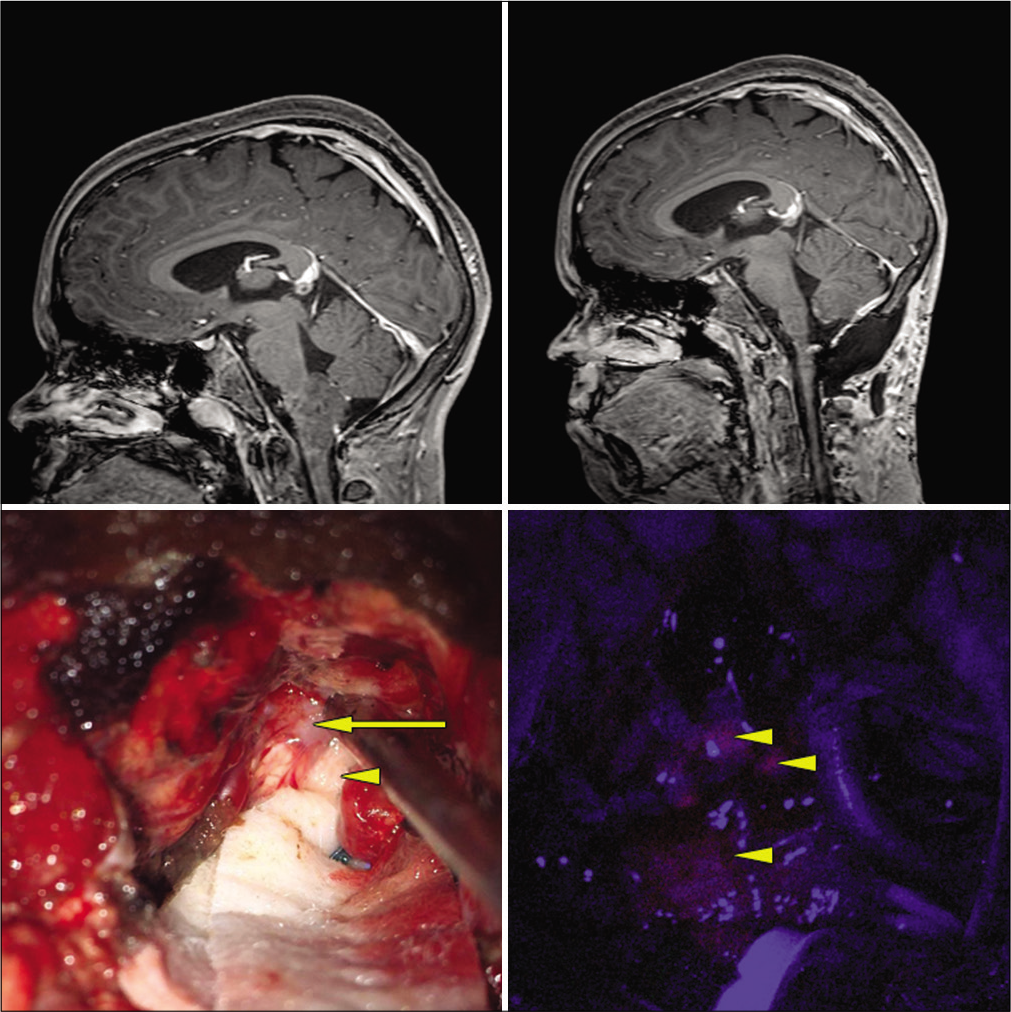
Figure 2:
Cervicomedullary pilocytic astrocytoma. Top left: preoperative, postgadolinium-enhanced sagittal MRI. Top right: postoperative gadolinium-enhanced MRI revealing GTR. Bottom left: intraoperative white light microscopic view. Arrow: obvious tumor under white light microscopy. Arrowhead: normal appearing tissue under white light microscopy. Bottom right: arrow heads: blue light mode reveals vague fluorescence of both obvious tumor and normal appearing area under white light mode.
The phenomenon of ventricle wall 5-ALA-induced fluorescence has been well described[
Unlike supratentorial malignant tumors, for which the extent of tumor resection (EOR) has a predictive value for outcome,[
We experienced no complications due to the administration of 5-ALA. All our postoperative deficits had resolved by the 3 months follow-up, and there were no significant changes in our postoperative LPS scores. It appears that the complication rate of 5-ALA in children is very low, with isolated reports of increased transaminases being the only complication directly attributable to 5-ALA administration.[
Limitations
The most significant limitation to our study was the unavailability of molecular testing as molecular subtyping is more likely to be predictive of fluorescence than any other single variable.[
An additional limitation inherent in our methodology is the considerable interobserver variability, discrepancies, and inconsistencies demonstrated by both radiologists and pediatric neurosurgeons in diagnosing DIPGs based on MRI imaging alone.[
At present, most studies quantify intraoperative fluorescence levels subjectively and suffer from intraobserver and interobserver variability.[
CONCLUSION
The extent of resection is an important prognostic factor[
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alvisi C, Cerisoli M, Maccheroni ME. Long-term results of surgically treated brainstem gliomas. Acta Neurochir (Wien). 1985. 76: 12-7
2. Barkovich AJ, Krischer J, Kun LE, Zimmerman RA, Freeman CR, Wara WM. Brain stem gliomas: A classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990. 91: 73-83
3. Beez T, Sarikay-Seiwert S, Steiger HJ, Hänggi D. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of brain tumors in children a technical report. Acta Neurosurg. 2014. 156: 597-604
4. Behnke J, Christen HJ, Mursch K, Markakis E. Intra-axial endophytic tumors in the pons and/or medulla oblongata. Childs Nerv Syst. 1997. 13: 135-46
5. Bonnin DA, Havrda MC, Lee MC, Evans L, Ran C, Qian DC. Characterizing the heterogeneity in 5-aminolevulinic acid-induced fluorescence in glioblastoma. J Neurosurg. 2019. 1: 1-9
6. Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C. Histopathological spectrum of pediatric diffuse intrinsic pontine glioma: Diagnostic and therapeutic implications. Acta Neuropathol. 2014. 128: 573-81
7. Burford C, Kayal N, Pandit A, Tailor J, Lavrador J, Bravo A. PP39. 5-Aminolevulinic acid aided resection of pediatric brain tumours: The UK’s first case series. Neuro Oncol. 2017. 19: i11
8. Chiang J, Diaz AK, Makepeace L, Li X, Han Y, Li Y. Clinical, imaging, and molecular analysis of pediatric pontine tumors lacking characteristic imaging features of DIPG. Acta Neuropathol Commun. 2020. 8: 57
9. Choux M, Lena G, Do L, Choux M, Di Rocco C, Hockley A.editors. Brainstem tumors. Pediatric Neurosurgery. New York: Churchill Livingstone; 2000. p. 471-91
10. Constantini S, Epstein F. Surgical Indication and Technical considerations in the management of benign brain stem gliomas. J Neurooncol. 1996. 28: 193-205
11. De Bonis P, Anile C, Pompucci A, Florentino A, Balducci M, Chiesa S. The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg. 2013. 115: 37-43
12. Dellaretti M, Câmara BB, Ferreira PH, da Silva Júnior JB, Arantes RM. Impact of histological diagnosis on the treatment of atypical brainstem lesions. Sci Rep. 2020. 10: 11065
13. Dellaretti M, Touzet G, Reyns N, Dubois F, Gusmão S, Pereira JL. Correlation among magnetic resonance imaging findings, prognostic factors for survival, and histological diagnosis of intrinsic brainstem lesions in children. J Neurosurg Pediatr. 2011. 8: 539-43
14. Epstein F, McCleary EL. Intrinsic brain-stem tumors of childhood: Surgical indications. J Neurosurg. 1986. 64: 11-5
15. Epstein FJ, Framer JP. Brain-stem glioma growth patterns. J Neurosurg. 1993. 78: 408-12
16. Ewelt C, Floeth FW, Felsberg J, Steiger HJ, Sabel M, Langen KJ. Finding the anaplastic focus in diffuse gliomas: The value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin Neurol Neurosurg. 2011. 113: 541-7
17. Fisher PG, Breiter SN, Carson BS, Wharam MD, Williams JA, Weingart JD. A clinicopathologic reappraisal of brain stem tumor classification. Cancer. 2000. 89: 1569-76
18. Frazier JL, Lee J, Thomale UW, Noggle JC, Cohen KJ, Jallo GI. Treatment of diffuse intrinsic brainstem gliomas: Failed approaches and future strategies. J Neurosurg Pediatr. 2009. 3: 259-69
19. Goryaynov SA, Widhalm G, Goldberg MF, Chelushkin D, Spallone A, Chernyshove KA. The role of 5-ALA in low grade gliomas and the influence of antiepileptic drugs on intraoperative fluorescence. Front Oncol. 2019. 9: 1-7
20. Grill J, Puget S, Andreiuolo F, Philippe C, MacConaill L, Kieran MW. Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer. 2012. 58: 489-91
21. Gupta N, Goumnerova LC, Manley P, Chi SN, Neuberg D, Puligandla M. Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol. 2018. 20: 1547-55
22. Hankinson TC, Campagna EJ, Foreman NK, Handler MH. Interpretation of magnetic resonance images in diffuse intrinsic pontine glioma: A survey of pediatric neurosurgeons. J Neurosurg Pediatr. 2011. 8: 97-102
23. Hayward RM, Patronas N, Baker EH, Vézina G, Albert PS, Warren KE. Inter-observer variability in the measurement of diffuse intrinsic pontine gliomas. J Neurooncol. 2008. 90: 57-61
24. Hefti M, Albert I, Luginbuelli V. Phenytoin reduces 5-aminolevulinic acid-induced protoporphyrin IX accumulation in malignant glioma cells. J Neurooncol. 2012. 108: 443-50
25. Hoffman HJ, Becker L, Craven MA. A clinically and pathologically distinct group of benign brain stem gliomas. Neurosurgery. 1980. 7: 243-8
26. Huang T, Garcia R, Qi J, Lulla R, Horbinski C, Behdad A. Detection of histone H3 K27M mutation and post-translational modifications in pediatric diffuse midline glioma via tissue immunohistochemistry informs diagnosis and clinical outcomes. Oncotarget. 2018. 9: 37112-24
27. Infinger LK, Stevenson CB. Re-examining the need for tissue diagnosis in pediatric diffuse intrinsic pontine gliomas: A review. Curr Neuropharmacol. 2017. 15: 129-33
28. Ishihara R, Katayama Y, Watanabe T, Yoshino A, Fukushima T, Sakatani K. Quantitative spectroscopic analysis of 5 aminolevulinic acid induced protoporphyrin IX fluorescence intensity in diffusely infiltration astrocytomas. Neurol Med Chir. 2007. 47: 53-7
29. Jaber M, Wölfer J, Ewelt C, Holling M, Hasselblatt M, Niederstadt T. The value of 5-aminolevulinic acid in low-grade gliomas and high-grade gliomas lacking glioblastoma imaging features: An analysis based on fluorescence, magnetic resonance imaging, 18F-fluoroethyl tyrosine positron emission tomography, and tumor molecular factors. Neurosurgery. 2016. 78: 401-11
30. Jallo GI, Biser-Rohrbaugh A, Freed D. Brainstem gliomas. Childs Nerv Syst. 2004. 20: 143-53
31. Kamp MA, Grosser P, Felsberg J. 5-Aminolevulinic acid (5-ALA) induced fluorescence in intracerebral metastases: A retrospective study. Acta Neurochir. 2012. 154: 223-8
32. Kamp MA, Molle ZK, Munoz-Bendix C, Rapp M, Sabel M, Steiger HJ. Various shades of red-A systematic analysis of qualitative estimation of ALA derived fluorescence in neurosurgery. Neurosurg Rev. 2018. 41: 3-18
33. Kaplan AM, Albright L, Zimmerman RA, Rorke LB, Li H, Moyett JM. Brainstem gliomas in children. Pediatr Neurosurg. 1996. 24: 185-92
34. Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018. 20: 123-31
35. Kestle J, Townsend JJ, Brockmeyer DL, Walker ML. Juvenile pilocytic astrocytoma of the brainstem in children. J Neurosurg Pediatr. 2014. 101: 1-6
36. Kim A, Khurana M, Moriyama Y, Wilson BC. Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements. J Biomed Opt. 2010. 15: 067006
37. Klimo P, Nesvick CL, Broniscer A, Orr BA, Choudhri AF. Malignant brainstem tumors in children, excluding diffuse intrinsic pontine gliomas. J Neurosurg Pediatr. 2016. 17: 57-65
38. Klimo P, Panandiker AS, Thompson CJ, Boop FA, Qaddoumi I, Gajjar A. Management and outcome of focal low-grade brainstem tumors in pediatric patients: The St. Jude experience. J Neurosurg Pediatr. 2013. 11: 274-81
39. Lansky SB, List MA, Lanskey LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer. 1987. 60: 1651-6
40. Lawrence JE, Steele CJ, Rovin RA, Belton RJ, Winn RJ. Dexamethasone alone and in combination with desipramine, phenytoin, valproic acid or levetiracetam interferes with 5 ALA mediated PpIX production and cellular retention in glioblastoma cells. J Neurooncol. 2016. 127: 15-21
41. Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus. 2014. 36: 1-10
42. Mauffrey C. Paediatric brainstem gliomas: Prognostic factors and management. J Clin Neurosci. 2006. 13: 431-7
43. Mehta VS, Chandra PS, Singh PK, Garg A, Rath GK. Surgical considerations for. “intrinsic” brainstem gliomas: Proposal of a modification in classification. Neurology India. 2009. 57: 274-81
44. Moon JH, Kim SH, Shim JK, Roh TH, Sung KS, Lee J. Histopathological implications of ventricle wall 5-aminolevulinic acid-induced fluorescence in the absence of tumor involvement on magnetic resonance images. Oncol Rep. 2016. 36: 837-44
45. Moreno RG, García LM, Bastidas HI, Tirado CA, Flores AM. Fluorescence guided surgery with 5-aminolevulinic acid for resection of spinal cord ependymomas. Asian Spine J. 2019. 13: 119-25
46. Morshed RA, Han SJ, Lau D, Berger MS. Wavelength-specific lighted suction instrumentation for 5-aminolevulinic acid fluorescence-guided resection of deep-seated malignant glioma: Technical note. J Neurosurg. 2018. 128: 1448-53
47. Motekallemi A, Jeltema HR, Metzemaekers JD, Van Dam GM, Crane LM, Groen RJ. The current status of 5-ALA fluorescence-guided resection of intracranial meningiomas a critical review. Neurosurg Rev. 2015. 38: 619-28
48. Ogiwara H, Morota N. The efficacy of a biopsy of intrinsic brainstem lesions for decision making of the treatments. Childs Nerv Syst. 2013. 29: 833-7
49. Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C. Extent of Resection in patients with glioblastoma: Limiting factors, perception of resectability, and effect of survival. J Neurosurg. 2012. 117: 851-9
50. Piccirillo SG, Spiteri I, Sottoriva A, Touloumis A, Ber S, Price SJ, Heywood R. Contributions to drug resistance in glioblastoma derived from malignant cells in the subependymal zone. Cancer Res. 2015. 75: 194-202
51. Pierre-Kahn A, Hirsch JF, Vinchon MD, Rayan C, Saint-Rose C, Renier D. Surgical management of brain-stem tumors in children results and statistical analysis of 75 cases. J Neurosurg. 1993. 79: 845-52
52. Pincus DW, Richter EO, Yachnis AT, Bennett J, Bhatti MT, Smith A. Brainstem stereotactic biopsy sampling in children. J Neurosurg. 2006. 104: 108-14
53. Pollack IF, Hoffman HJ, Humphreys RP, Becker L. The long-term outcome after surgical treatment of dorsally exophytic brain-stem gliomas. J Neurosurg. 1993. 78: 859-63
54. Preuß M, Renner C, Krupp W, Christiansen H, Fischer L, Merkenschlager A. The use of 5 aminolevulinic acid fluorescence guidance in resection of pediatric brain tumors. Childs Nerv Syst. 2013. 29: 1263-7
55. Puppa AD, Rustemi O, Gioffré G, Scienza R. Approaching a brainstem high-grade glioma (HGG) with the assistance of 5-aminolevulinic acid (5-ALA) technology: A new strategy for an old surgical challenge. Neurol Sci. 2014. 36: 797-9
56. Puppa AD, Rustemi O, Gioffrè G, Troncon I, Lombardi G, Rolma G. Predictive value of intraoperative 5-aminolevulinic acid-induced fluorescence for detecting bone invasion in meningioma surgery. J Neurosurg. 2014. 120: 840-5
57. Rachinger W, Grau S, Holtmannspötter M, Herms J, Tonn JC, Kreth FW. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry. 2009. 80: 1134-9
58. Rapp M, Kamp M, Steiger HJ, Sabel M. Endoscopic-assisted visualization of 5 aminolevulinic acid induced fluorescence in malignant glioma surgery: A technical note. World Neurosurg. 2014. 82: 1-3
59. Recinos PF, Sciubba DM, Jallo GI. Brainstem tumors: Where are we today?. Pediatr Neurosurg. 2007. 43: 192-201
60. Ritz R, Feigl GC, Schuhmann MU, Ehrhardt A, Danz S, Noell S. Use of 5-ALA fluorescence guided endoscopic biopsy of a deep-seated primary malignant brain tumor. J Neurosurg. 2011. 114: 1410-3
61. Ritz R, Scheidle C, Noell S, Roser F, Schenk M, Dietz K. In vitro comparison of hypericin and 5-aminolevulinic acid-derived protoporphyrin IX for photodynamic inactivation of medulloblastoma cells. PLoS One. 2012. 7: e51974
62. Roberts DW, Valdés PA, Harris BT, Fontaine KM, Hartov A, Fan X. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between 5-aminolevulinic acid induced protoporphyrin IX Fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J Neurosurg. 2011. 114: 595-603
63. Robertson PL, Allen JC, Abbott IR, Miller DC, Fidel J, Epstein FJ. Cervicomedullary tumors in children: A distinct subset of brainstem gliomas. Neurology. 1994. 44: 1798-803
64. Roth J, Constantini S. 5ALA in pediatric brain tumors is not routinely beneficial. Childs Nerv Syst. 2017. p. 850-9
65. Saito K, Hirai T, Takeshima H, Kadota Y, Yamashita S, Ivanova A. Genetic factors affecting intraoperative 5-aminolevulinic acid-induced fluorescence of diffuse gliomas. Radiol Oncol. 2017. 51: 142-50
66. Samkoe KS, Gibbs-Strauss SL, Yang HH, Hekmatyar SK, Hoopes PJ. Protoporphyrin IX Fluorescence contrast in invasive glioblastomas is linearly correlated with Gd enhanced magnetic resonance image contrast but has diagnostic accuracy. J Biomed Opt. 2011. 16: 096008
67. Sanai N, Snyder LA, Honea NJ, Coons SW, Eschbacher JM, Smith KA. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011. 115: 740-8
68. Sandri A, Sardi N, Genitori L, Peretta P, Basson ME, Bertin D. Diffuse and focal brain stem tumors in childhood: Prognostic factors and surgical outcome. Childs Nerv Syst. 2006. 22: 1127-35
69. Schild SE, Stafford SL, Brown PD, Wood CP, Scheithauer BW, Schomberg PJ. The results of radiotherapy for brainstem tumors. J Neurooncol. 1998. 40: 171-7
70. Schwake M, Günes D, Köchling M, Brentrup A. Kinetics of porphyrin fluorescence accumulation in pediatric brain tumor cells incubated in 5-aminolevulinic acid. Acta Neurochir (Wien). 2014. 156: 1077-84
71. Schwake M, Nemes A, Dondrop J, Schroeteler J, Schipmann S, Senner V. In-vitro use of 5-ALA for photodynamic therapy in pediatric brain tumors. Neurosurgery. 2018. 83: 1328-37
72. Schwake M, Schipmann S, Müther M, Köchling M, Brentrup A, Stummer W. 5-ALA fluorescence-guided surgery in pediatric brain tumors a systematic review. Acta Neurochir. 2019. 161: 1099-108
73. Stroink AR, Harold MD, Hoffman J, Hendrick EB, Humphreys RP. Diagnosis and management of pediatric brainstem gliomas. J Neurosurg. 1986. 65: 745-50
74. Stummer W, Koch R, Valle RD, Roberts DW, Sanai N, Kalkanis S. Intraoperative fluorescence diagnosis in the brain: A systematic review and suggestions for future standards on reporting diagnostic accuracy and clinical utility. Acta Neurochir (Wien). 2019. 161: 2083-98
75. Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid induced porphyrins: A prospective study in 52 consecutive patients. J Neurosurg. 2000. 93: 1003-13
76. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulan HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomized controlled multicentre phase III trial. Lancet Oncol. 2006. 7: 392-401
77. Stummer W, Rodriques F, Schucht P, Preuss M, Wiewrodt D, Nestler U. Predicting the “usefulness” of 5-ALA derived tumor fluorescence for fluorescence guided resections in pediatric brain tumors: A European survey. Acta Neurochir. 2014. 156: 2315-24
78. Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A. 5 aminolevulinic acid derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014. 74: 310-20
79. Tejada S, Aquilina K, Goodden J, Pettorini B, Mallucci C, van Veelen ML. Biopsy in diffuse pontine gliomas: Expert neurosurgeon opinion-a survey from the SIOPE brain tumor group. Childs Nerv Syst. 2020. 36: 705-11
80. Teo C, Siu TL. Radical resection of focal brainstem gliomas: Is it worth doing?. Childs Nerv Syst. 2008. 24: 1307-14
81. Tomita T, McLone DG, Naidich TP. Brain stem gliomas in childhood. J Neuro Oncol. 1984. 2: 117-22
82. Utsuki S, Oka H, Sato S, Shimizu S, Suzuki S, Tanizaki Y. Histological examination of false positive tissue resection using 5 aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir. 2007. 47: 210-4
83. Valés PA, Jacobs V, Harris BT, Wilson BC, Leblond F, Paulson KD. Quantitative fluorescence using 5-aminolevulinic acid induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J Neurosurg. 2015. 123: 771-80
84. Wei L, Fujita Y, Sanai N, Liu JT. Toward quantitative neurosurgical guidance with high-resolution microscopy of 5-aminolevulinic acid-induced protoporphyrin IX. Front Oncol. 2019. 9: 592
85. Weiner HL, Freed D, Woo HH, Rezai AR, Kim R, Epstein FJ. Intra-axial tumors of the cervicomedullary junction: Surgical results and long term outcome. Pediatr Neurosurg. 1997. 27: 12-8
86. Widhalm G, Kiesel B, Weshrer A, Weldinger TT, Preusser M, Marosi C. 5-aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast enhancement. PLoS One. 2013. 8: 1-8
87. Xue Z, Kong L, Pan C, Wu Z, Zhang J, Zhang L. Fluorescein-guided surgery for pediatric brainstem gliomas: Preliminary study and technical notes. J Neurol Surg B. 2018. 79: 340-6
88. Yin L, Zhang L. Correlation between MRI findings and histological diagnosis of brainstem glioma. Can J Neurol Sci. 2013. 40: 348-54
89. Zhang C, Boop FA, Ruge J. The use of 5-aminolevulinic acid in resection of pediatric brain tumors: A critical review. J Neurooncol. 2019. 141: 567-73