- Department of Neurosurgery, The Ohio State University Wexner Medical Center, Columbus, Ohio, United States.
- Department of Radiation Oncology, The Ohio State University Wexner Medical Center, Columbus, Ohio, United States.
- Department of Internal Medicine, The Ohio State University Wexner Medical Center, Columbus, Ohio, United States.
Correspondence Address:
Mark A. Damante
Department of Neurosurgery, The Ohio State University Wexner Medical Center, Columbus, Ohio, United States.
DOI:10.25259/SNI_554_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mark A. Damante1, Kristin M. Huntoon1, Joshua D. Palmer1,2, David A. Liebner3, James Bradley Elder1. A case of multiple synchronously diagnosed brain metastases from alveolar soft part sarcoma without concurrent lung involvement. 24-Mar-2021;12:111
How to cite this URL: Mark A. Damante1, Kristin M. Huntoon1, Joshua D. Palmer1,2, David A. Liebner3, James Bradley Elder1. A case of multiple synchronously diagnosed brain metastases from alveolar soft part sarcoma without concurrent lung involvement. 24-Mar-2021;12:111. Available from: https://surgicalneurologyint.com/surgicalint-articles/10668/
Abstract
Background: Alveolar soft part sarcoma (ASPS) is a rare soft-tissue sarcoma with a propensity for early hematogenous dissemination to the lungs and frequent brain metastasis. The development of lung metastasis almost invariably precedes intracranial involvement. There are no previously reported cases in which a patient was synchronously diagnosed with ASPS and multiple brain metastasis without lung involvement.
Case Description: A 29-year-old gentleman was found to have three intracranial lesions following the onset of generalized seizures. Staging studies identified a soft-tissue mass in the left thigh and an adjacent femoral lesion. Biopsy of the soft-tissue mass was consistent with ASPS. The patient then underwent neoadjuvant stereotactic radiotherapy to all three brain lesions, followed by en bloc resection of the dominant lesion. The patient was then started on a programmed death-ligand 1 (PD-L1) inhibitor. Subsequent surgical resection of the primary lesion and femur metastasis demonstrates a histopathologic complete response of the bony metastasis and partial response of the primary lesion. At present, the patient has received 14 cycles of atezolizumab without recurrence of the primary or bony lesions and the irradiated intracranial disease has remained stable without recurrence of the resected dominant lesion.
Conclusion: While intracranial involvement is relatively common in ASPS, a case with multiple, synchronously diagnosed brain metastasis without concurrent lung metastasis has not been described. The presented case discusses the safety and efficacy of aggressive management of intracranial disease in the setting of atezolizumab. Prospective evaluation of the efficacy of checkpoint inhibitors and the prognostic value of PD-L1 expression in ASPS with brain metastasis are necessary.
Keywords: Abscopal effect, Brain metastasis, Checkpoint inhibitor, Immunotherapy, Sarcoma, Stereotactic radiotherapy
INTRODUCTION
Alveolar soft part sarcoma (ASPS) accounts for roughly 1% of all soft-tissue sarcomas (STSs). ASPS typically presents as a slow-growing mass in the lower extremity. Detection of the nonreciprocal translocation t (X; 17) (p11.2; q25) between chromosome 17q25 and Xp11 resulting in the chimeric ASPSCR1-transcription factor E3 (TFE3) fusion gene is diagnostic. The standard of care includes wide margin, function-sparing, excision of the primary lesion, often with adjuvant radiation therapy (RT) in the form of external beam RT or brachytherapy.[
ASPS has a propensity for early, often synchronous, hematogenous dissemination to the lungs and is diagnosed in 63% of cases.[
The role of systemic therapy for metastatic ASPS is poorly understood.[
The presented case describes the clinical and surgical management of a patient with synchronous brain metastases from ASPS without definitive evidence of concurrent lung metastasis, which is exceptionally rare, with similarities to only one prior case.[
CASE DESCRIPTION
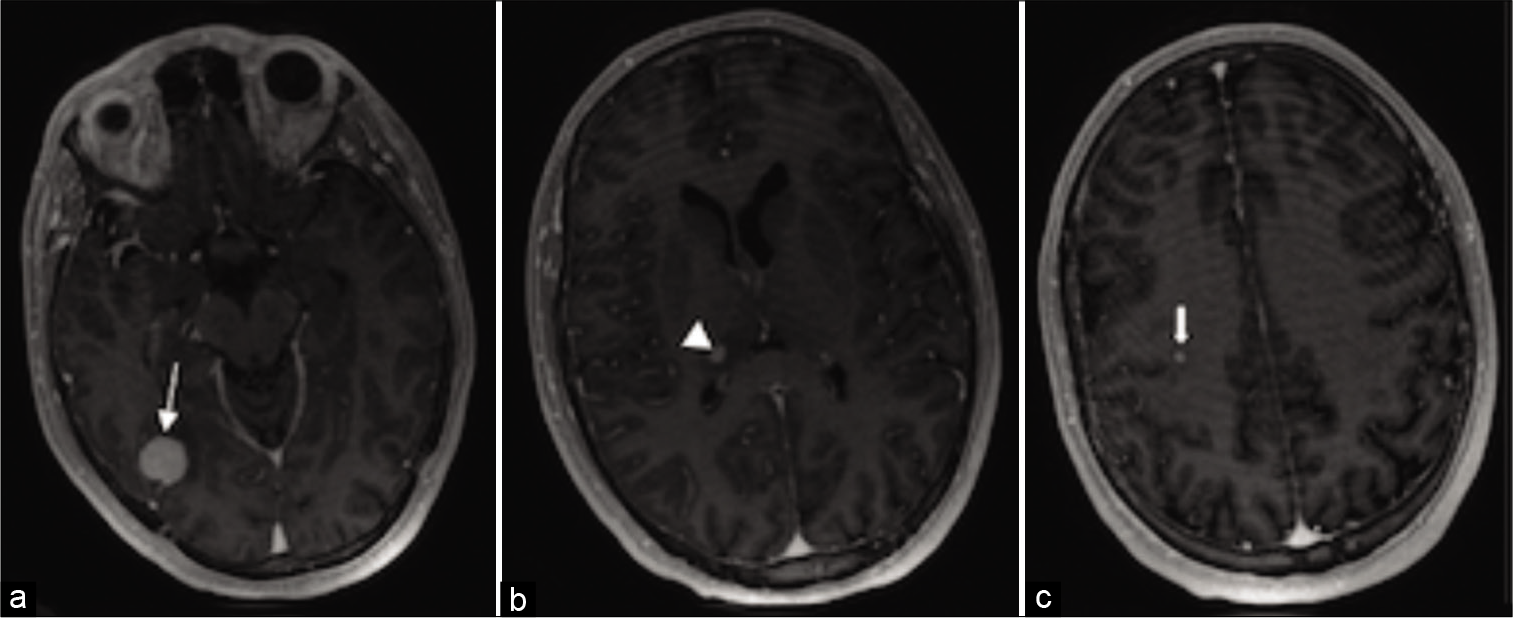
A 29-year-old gentleman with no significant medical history presented following a generalized tonic-clonic seizure. His physical examination and vital signs were unremarkable except for a grossly evident left lateral thigh mass. MRI brain showed three contrast-enhancing lesions: 14 × 6 mm right posterior temporal lesion, 7 × 5 mm posteromedial right thalamic lesion, and 3 mm subcortical posterior right frontal lobe lesion [
Figure 2:
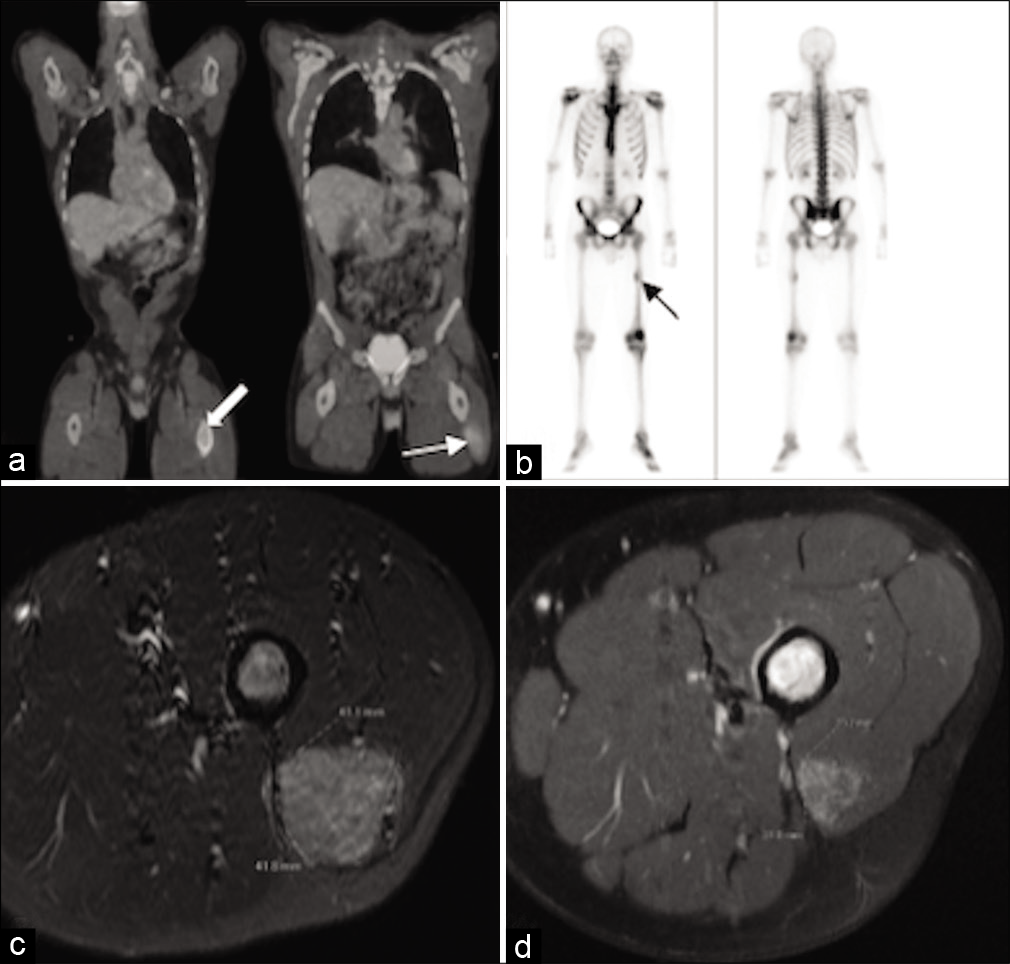
Pretreatment PET scan and MRI femur. There are hypermetabolic lesions of the left femur (block/black arrow) and vastus lateralis (arrow) (a and b). No radiotracer uptake is noted within the lungs (a). MRI femur depicted a 4.2 cm × 4.1 cm vastus lateralos lesion (c) which, following 7 atezolizumab cycles, regressed to 2.3 cm × 2.1 cm (d).
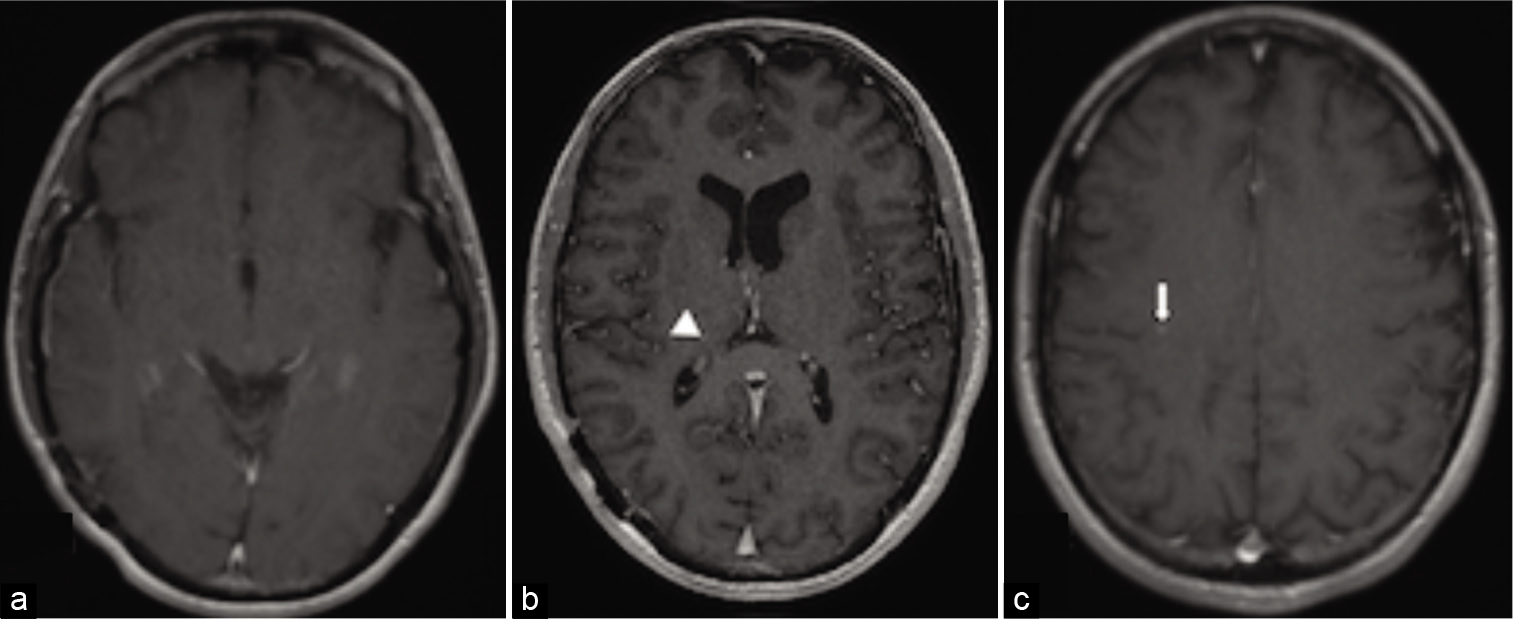
In <1 month from initial diagnosis, the dominant posterior temporal lesion grew from 14 mm to 22 mm in axial diameter. After interdisciplinary discussion, the patient agreed to neoadjuvant stereotactic radiotherapy (SRT) (24 Gy in three fractions)[
Atezolizumab was started 2 weeks after surgery (1200 mg dose every 21-day cycle). After 4 cycles, PET imaging showed continued radiotracer uptake in the left vastus lateralis and femur. MRI brain at that time showed no evidence of recurrence in the postoperative bed, a PR and reduced T2-FLAIR signal of the thalamic lesion and stable appearance of the posterior frontal lobe lesion (RECIST 1.1). Following 7 cycles, repeat MRI femur showed a PR of the primary lesion from 4.2 × 4.1 cm to 2.3 × 2.1 cm (RECIST 1.1) [
DISCUSSION
The presented report describes the first definitive case of ASPS synchronously diagnosed with multiple brain metastases and no concurrent lung metastasis. In a retrospective review of 70 patients with ASPS, 48 patients (65%) were diagnosed with Stage IV disease.[
Important deviations from the presented case include the fact that lung involvement was evaluated with chest X-ray only, which has a lower sensitivity for metastases than CT chest, the current standard of care in ASPS.[
The response to ICI is currently being determined by multiple clinical trials. A Phase II trial evaluating the efficacy of atezolizumab in the treatment of metastatic ASPS is currently active, however, patients with symptomatic intracranial disease are excluded (NCT03141684).[
In some STS subtypes, PD-L1 expression is associated with higher tumor stage, deep-seated sarcoma, distant metastasis, higher histologic grade, tumor differentiation, and tumor necrosis.[
The role of ICI with concurrent radiation for STS is being evaluated by the NEXIS trial (NCT03116529), though patients with symptomatic or uncontrolled brain metastases are excluded.[
In addition to a concurrent ICI and SRT strategy, the authors also employed a relatively new treatment paradigm involving neoadjuvant SRT followed by surgical resection of the dominant lesion. As described first by Asher et al., potential benefits of neoadjuvant radiation are 3-fold: (1) a better-defined treatment target thus diminishing the volume of healthy parenchyma exposed to radiation and decreasing risk for radiation necrosis, (2) the sterilization of tumor cells preoperatively may decrease the risk of intraoperative dissemination and leptomeningeal seeding, and (3) an increase in tumor antigen exposure, which may further enhance ICI response.[
CONCLUSION
The presented case provides a unique presentation of ASPS with multiple, synchronous brain metastases without evidence of lung metastasis. Aggressive local management of brain metastases with surgery and SRT, in addition to ongoing ICI followed by surgical resection of the primary lesion, has thus far led to disease control. This report supports aggressive local therapy including surgery and SRT for symptomatic brain metastases from ASPS. Patients with similar presentations may warrant consideration for clinical trials given the rarity of metastatic ASPS and the potential for good outcomes with aggressive, early treatment of brain metastases. Additional studies are necessary to clarify optimal management strategies for patients with ASPS brain metastases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Asher AL, Burri SH, Wiggins WF, Kelly RP, Boltes MO, Mehrlich M. A new treatment paradigm: Neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014. 88: 899-906
2. Chen AP.editors. Testing Atezolizumab in People with Advanced Alveolar Soft Part Sarcoma. 2020. p.
3. D’Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015. 46: 357-65
4. Groisberg R, Hong DS, Behrang A, Hess K, Janku F, Piha-Paul S. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J Immunother Cancer. 2017. 5: 100
5. Howard BA, Rubenstein JD, Lewis AJ. Case report 371: Alveolar soft parts sarcoma (brain and thigh). Skeletal Radiol. 1986. 15: 468-72
6. Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One. 2013. 8: e82870
7. Lewin J, Davidson S, Anderson ND, Lau BY, Kelly J, Tabori U. Response to immune checkpoint inhibition in two patients with alveolar soft-part sarcoma. Cancer Immunol Res. 2018. 6: 1001-7
8. McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016. 2: 46-54
9. Mitsis D, Francescutti V, Skitzki J. Current immunotherapies for sarcoma: Clinical trials and rationale. Sarcoma. 2016. 2016: 9757219
10. Ng VY.editors. Neoadjuvant Durvalumab and Tremelimumab Plus Radiation for High Risk Soft-Tissue Sarcoma (NEXIS). 2020. p.
11. Palmer JD, Sebastian NT, Chu J, DiCostanzo D, Bell EH, Grecula J. Single-isocenter multitarget stereotactic radiosurgery is safe and effective in the treatment of multiple brain metastases. Adv Radiat Oncol. 2020. 5: 70-6
12. Paoluzzi L, Maki RG. Diagnosis, prognosis, and treatment of alveolar soft-part sarcoma: A review. JAMA Oncol. 2019. 5: 254-60
13. Patel KR, Martinez A, Stahl JM, Logan SJ, Perricone AJ, Ferris MJ. Increase in PD-L1 expression after pre-operative radiotherapy for soft tissue sarcoma. Oncoimmunology. 2018. 7: e1442168
14. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015. 14: 847-56
15. Portera CA, Ho V, Patel SR, Hunt KK, Feig BW, Respondek PM. Alveolar soft part sarcoma: Clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer. 2001. 91: 585-91
16. Prabhu RS, Patel KR, Press RH, Soltys SG, Brown PD, Mehta MP.editors. Preoperative vs postoperative radiosurgery for resected brain metastases: A review. Neurosurgery. 2019. 84: 19-29
17. Ramu EM, Houdek MT, Isaac CE, Dickie CI, Ferguson PC, Wunder JS. Management of soft-tissue sarcomas; treatment strategies, staging, and outcomes. SICOT J. 2017. 3: 20
18. Rekhi B, Ingle A, Agarwal M, Puri A, Laskar S, Jambhekar NA. Alveolar soft part sarcoma revisited: Clinicopathological review of 47 cases from a tertiary cancer referral centre, including immunohistochemical expression of TFE3 in 22 cases and 21 other tumours. Pathology. 2012. 44: 11-7
19. Salvati M, Cervoni L, Caruso R, Gagliardi FM, Delfini R. Sarcoma metastatic to the brain: A series of 15 cases. Surg Neurol. 1998. 49: 441-4
20. Tao X, Hou Z, Wu Z, Hao S, Liu B. Brain metastatic alveolar soft-part sarcoma: Clinicopathological profiles, management and outcomes. Oncol Lett. 2017. 14: 5779-84
21. Wang CH, Lee N, Lee LS. Successful treatment for solitary brain metastasis from alveolar soft part sarcoma. J Neurooncol. 1995. 25: 161-6
22. Wilky BA, Trucco MM, Subhawong TK, Florou V, Park W, Kwon D. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019. 20: 837-48
23. Williams A, Bartle G, Sumathi VP, Meis JM, Mangham DC, Grimer RJ. Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: Useful diagnostic tools in cases with unusual histological features. Virchows Arch. 2011. 458: 291-300