- Department of Neurosurgery, Kohnan Hospital, Sendai, Japan
- Department of Neurosurgery, Akita University Graduate School of Medicine, Akita, Japan
- Department of Neurosurgery, Tohoku University Graduate School of Medicine, Sendai, Japan
- Department of Pathology, Tohoku University Graduate School of Medicine, Sendai, Japan
Correspondence Address:
Hidenori Endo
Department of Neurosurgery, Tohoku University Graduate School of Medicine, Sendai, Japan
DOI:10.4103/2152-7806.173319
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abe T, Endo H, Shimizu H, Fujimura M, Endo T, Sakata H, Watanabe M, Tominaga T. A case of ruptured infectious anterior cerebral artery aneurysm treated by interposition graft bypass using the superficial temporal artery. Surg Neurol Int 06-Jan-2016;7:5
How to cite this URL: Abe T, Endo H, Shimizu H, Fujimura M, Endo T, Sakata H, Watanabe M, Tominaga T. A case of ruptured infectious anterior cerebral artery aneurysm treated by interposition graft bypass using the superficial temporal artery. Surg Neurol Int 06-Jan-2016;7:5. Available from: http://surgicalneurologyint.com/surgicalint_articles/a-case-of-ruptured-infectious-anterior-cerebral-artery-aneurysm-treated-by-interposition-graft-bypass-using-the-superficial-temporal-artery/
Abstract
Background:To describe the application of an interposition graft bypass using superficial temporal artery (STA) for the treatment of a ruptured anterior cerebral artery (ACA) infectious aneurysm.
Case Description:A 30-year-old male suffered from severe headache with high fever. The patient's diagnosis was ruptured infectious ACA aneurysm at the A3 segment with a maximum diameter of 4.5 mm, caused by infectious endocarditis. The patient was initially treated with high-dose intravenous antibiotics. Follow-up digital subtraction angiography (DSA) revealed that the fusiform aneurysm had enlarged to a maximum diameter of 14.0 mm. A left paracentral artery, supplying the motor area of the left lower extremity, originated from the body of this aneurysm. Because the angiographic findings suggested a risk of recurrent bleeding, the patient underwent open surgery. Interposition graft bypass using the STA was performed to reconstruct the left A3 segment in an end-to-side manner (left proximal callosomarginal artery – STA graft – left distal pericallosal artery). Then, the origin of the left paracentral artery was cut and anastomosed to the STA graft in an end-to-side manner. The affected parent artery was trapped, and the aneurysm was resected. Postoperative magnetic resonance imaging showed no ischemic or hemorrhagic complications, and postoperative DSA revealed the patency of the interposition graft. Pathological diagnosis of the resected aneurysm revealed features corresponding to infectious cerebral aneurysm. The postoperative course was uneventful, and the patient was discharged without any neurological deficits.
Conclusion:In the treatment of infectious cerebral aneurysms, revascularization should be considered when the affected artery supplies the eloquent area. Interposition graft bypass using the STA is one of the options for revascularization surgery for the treatment of infectious ACA aneurysms.
Keywords: Anterior cerebral artery, infectious cerebral aneurysm, interposition graft bypass, subarachnoid hemorrhage, superficial temporal artery
INTRODUCTION
Infectious cerebral aneurysm is relatively rare, accounting for 0.7–6.5% of all intracranial (IC) aneurysms.[
CASE REPORT
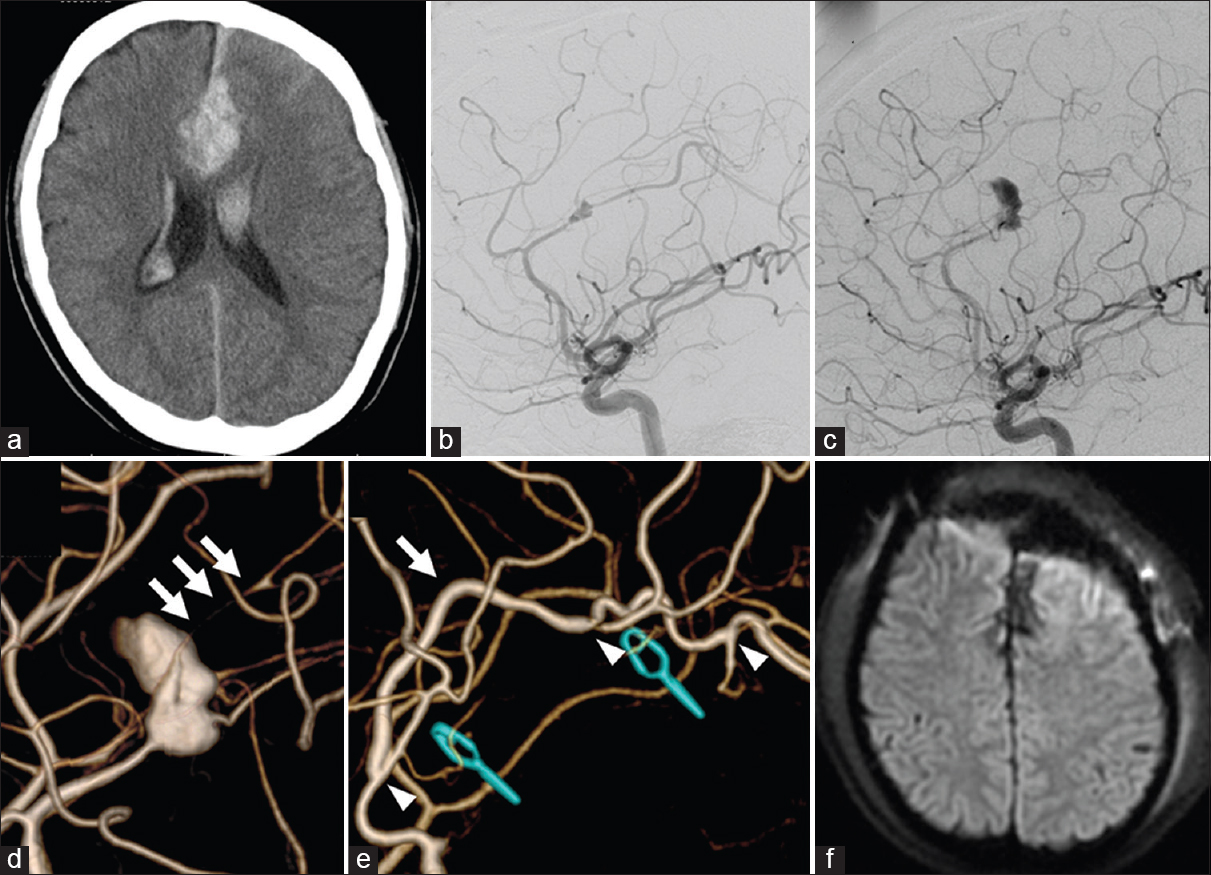
A 30-year-old male was admitted to our hospital with complaints of a 3-day history of severe headache and high fever. On admission, he presented with disorientation, and his body temperature was higher than 39°C. Blood examination showed a white blood cell count of 12,200 cells/mm3 and C-reactive protein of 1.22 mg/dl. A computed tomography scan revealed subarachnoid hemorrhage (SAH) with intracerebral hematoma, predominantly in the anterior interhemispheric fissure, and intraventricular hematoma [
Figure 1
Primary computed tomography revealing subarachnoid hemorrhage and intracerebral hematoma predominantly in the anterior interhemispheric fissure (a). Initial digital subtraction angiography demonstrating a fusiform aneurysm of the left pericallosal artery (b). Follow-up digital subtraction angiography performed 20 days after the bleeding revealing enlargement of the aneurysm (c). Three-dimensional digital subtraction angiography indicating the paracentral artery originating from the aneurysmal sac (triple arrows) (d). Postoperative digital subtraction angiography showing disappearance of the aneurysm and patency of the interposition graft bypass. An arrow indicates the graft bypass. Left and right arrowheads indicate the anastomosis sites of the callosomarginal artery-superficial temporal artery-pericallosal artery bypass. Middle arrowhead indicates the anastomosis site of the superficial temporal artery-paracentral artery (e). Postoperative diffusion-weighted imaging showing no ischemic complications (f)
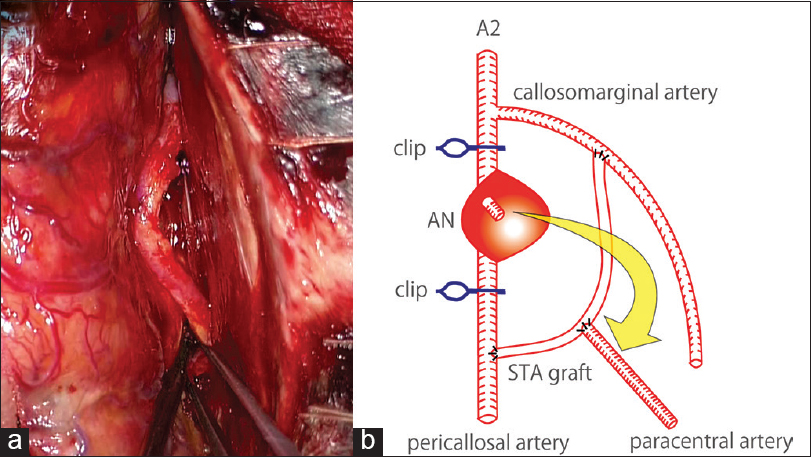
Left frontal craniotomy was performed, and a proximal side of the A2 segment was initially found within the anterior interhemispheric fissure. We distally followed the ACA, and subsequently observed an old SAH. Then, we found the distal part of the A3 segment before approaching to the aneurysm. Considering the risk of premature rupture, we decided to create a bypass in advance of the dissection of the aneurysm. A frontal branch of the left STA, 8 cm in length, was harvested, and this STA was used as an interposition graft to reconstruct the left ACA in an end-to-side manner: Left callosomarginal artery (proximal side) – STA graft – left pericallosal artery (distal side) [Figure
Hematoxylin-eosin stained sections and Elastica–Masson stained sections of the resected aneurysm corresponded to the features of an infectious cerebral aneurysm [
Figure 3
H- and E-stained section of the resected aneurysm revealing features of a ruptured infectious pseudo-aneurysm (a). The arrows show vascular intima partially absent in an Elastica–Masson stained section (b). The adventitia of the resected anterior cerebral artery was invaded by many inflammatory cells in an H- and E-stained section (c)
DISCUSSION
The most common cause of infectious cerebral aneurysms is IE; approximately 65% of such cases are associated with IE.[
The optimal treatment strategy for infectious cerebral aneurysm is still controversial because of its rarity. It is generally considered possible to manage unruptured infectious aneurysms with the administration of antibacterial agents and sequential angiographic follow-up.[
In the present case, resection of the infectious ACA aneurysm with interposition graft bypass using the STA was successfully achieved without ischemic or hemorrhagic complications. In a previous report, ACA aneurysms requiring bypass were found to be rare.[
For infectious cerebral aneurysms, the safety of an interposition graft bypass is unknown. There have been only two reported cases treated with IC-IC interposition graft bypass.[
CONCLUSION
Revascularization should be considered when the affected artery supplies the eloquent area in the treatment of infectious cerebral aneurysms. In this setting, interposition graft bypass using the STA is one of the options for revascularization surgery in the ACA territory.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abla AA, Lawton MT. Anterior cerebral artery bypass for complex aneurysms: An experience with intracranial-intracranial reconstruction and review of bypass options. J Neurosurg. 2014. 120: 1364-77
2. Appelboom G, Kadri K, Hassan F, Leclerc X. Infectious aneurysm of the cavernous carotid artery in a child treated with a new-generation of flow-diverting stent graft: Case report. Neurosurgery. 2010. 66: E623-4
3. Bohmfalk GL, Story JL, Wissinger JP, Brown WE. Bacterial intracranial aneurysm. J Neurosurg. 1978. 48: 369-82
4. Chapot R, Houdart E, Saint-Maurice JP, Aymard A, Mounayer C, Lot G. Endovascular treatment of cerebral mycotic aneurysms. Radiology. 2002. 222: 389-96
5. Chun JY, Smith W, Halbach VV, Higashida RT, Wilson CB, Lawton MT. Current multimodality management of infectious intracranial aneurysms. Neurosurgery. 2001. 48: 1203-13
6. Ducruet AF, Hickman ZL, Zacharia BE, Narula R, Grobelny BT, Gorski J. Intracranial infectious aneurysms: A comprehensive review. Neurosurg Rev. 2010. 33: 37-46
7. Gelfenbeyn M, Natarajan SK, Sekhar LN. Large distal anterior cerebral artery aneurysm treated with resection and interposition graft: Case report. Neurosurgery. 2009. 64: E1008-9
8. Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious aneurysm): Current options for diagnosis and management. Neurocrit Care. 2009. 11: 120-9
9. Kuo I, Long T, Nguyen N, Chaudry B, Karp M, Sanossian N. Ruptured intracranial mycotic aneurysm in infective endocarditis: A natural history. Case Rep Med 2010. 2010. p.
10. Matsubara N, Miyachi S, Izumi T, Yamanouchi T, Asai T, Ota K. Results and current trends of multimodality treatment for infectious intracranial aneurysms. Neurol Med Chir (Tokyo). 2015. 55: 155-62
11. Molinari GF, Smith L, Goldstein MN, Satran R. Pathogenesis of cerebral mycotic aneurysms. Neurology. 1973. 23: 325-32
12. Mura J, Riquelme F, Cuevas JL, Luna F, Vizhñay P. Simplified azygos anterior cerebral bypass: Y-shaped superficial temporal artery interposition graft from A2 with double reimplantation of pericallosal arteries: Technical case report. Neurosurgery. 2013. 72: OnsE235-40
13. Nussbaum L, Defillo A, Zelensky A, Nussbaum ES. A short segment intracranial-intracranial jump graft bypass followed by proximal arterial occlusion for a distal MCA aneurysm. Surg Neurol Int. 2011. 2: 98-
14. Peters PJ, Harrison T, Lennox JL. A dangerous dilemma: Management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis. 2006. 6: 742-8
15. Phuong LK, Link M, Wijdicks E. Management of intracranial infectious aneurysms: A series of 16 cases. Neurosurgery. 2002. 51: 1145-51
16. Terasaka S, Satoh M, Echizenya K, Murai H, Fujimoto S, Asaoka K. Revascularization of the anterior cerebral artery using a free superficial temporal artery graft: A case report. Surg Neurol. 1997. 48: 164-9
17. Yen PS, Teo BT, Chen SC, Chiu TL. Endovascular treatment for bilateral mycotic intracavernous carotid aneurysms.Case report and review of the literature. J Neurosurg. 2007. 107: 868-72
18. Yokoh A, Ausman JI, Dujovny M, Diaz FG, Berman SK, Sanders J. Anterior cerebral artery reconstruction. Neurosurgery. 1986. 19: 26-35