- Department of Neurosurgery, Minamata City General Hospital and Medical Center, Kumamoto,
- Department of Neurosurgery, Miyazaki Prefectural Nobeoka Hospital, Nobeoka,
- Department of Neurosurgery, Kumamoto University Hospital, Kumamoto, Japan.
Correspondence Address:
Takaho Tokuda, Department of Neurosurgery, Minamata City General Hospital and Medical Center, Kumamoto, Japan.
DOI:10.25259/SNI_282_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takaho Tokuda1, Seiji Tajiri1, Yutaka Ueda2, Yuki Ohmori3, Akitake Mukasa3. A case of subarachnoid hemorrhage caused by multiple cerebral aneurysms due to segmental arterial mediolysis. 29-Apr-2022;13:175
How to cite this URL: Takaho Tokuda1, Seiji Tajiri1, Yutaka Ueda2, Yuki Ohmori3, Akitake Mukasa3. A case of subarachnoid hemorrhage caused by multiple cerebral aneurysms due to segmental arterial mediolysis. 29-Apr-2022;13:175. Available from: https://surgicalneurologyint.com/surgicalint-articles/11568/
Abstract
Background: Segmental arterial mediolysis (SAM) is a condition in which an aneurysm is formed by causing lysis of the media and remodeling of blood vessels. Short-term recurrence has been reported in abdominal aortic aneurysms. Cerebral aneurysms have been suggested to form in a short period not only in the abdominal cavity but also in the intracranial arteries in SAM.
Case Description: A 36-year-old pregnant woman at 35 weeks’ gestation developed sudden headache and disorientation. Head magnetic resonance imaging showed a small amount of subarachnoid hemorrhage in the right ambient cistern. A fusiform cerebral aneurysm was found in the periphery of the right superior cerebellar artery, and small saccular aneurysms were found in the periphery of the right posterior cerebral artery and left posterior inferior cerebral artery. After delivery of the fetus, endovascular embolization of the ruptured aneurysm was performed. However, 10-week postoperatively, she developed sudden headache. Hemorrhage was found in the fourth ventricle, and enlargement of the left posterior inferior cerebellar artery (PICA) peripheral aneurysm and disappearance of the right posterior cerebral artery peripheral aneurysm were confirmed. A ruptured aneurysm in the peripheral left PICA was removed after trapping. Intraoperatively, an unruptured thrombosed aneurysm that was not visualized by imaging was also removed. Histopathological examination showed no calcification or inflammation, rupture of the internal elastic lamina, and lack of segmentation, and SAM was diagnosed.
Conclusion: In atypical dissecting aneurysms, SAM should be considered as a differential diagnosis. Systemic examination and short-term follow-up are also necessary.
Keywords: Dissecting aneurysm, Segmental arterial mediolysis, Subarachnoid hemorrhage
INTRODUCTION
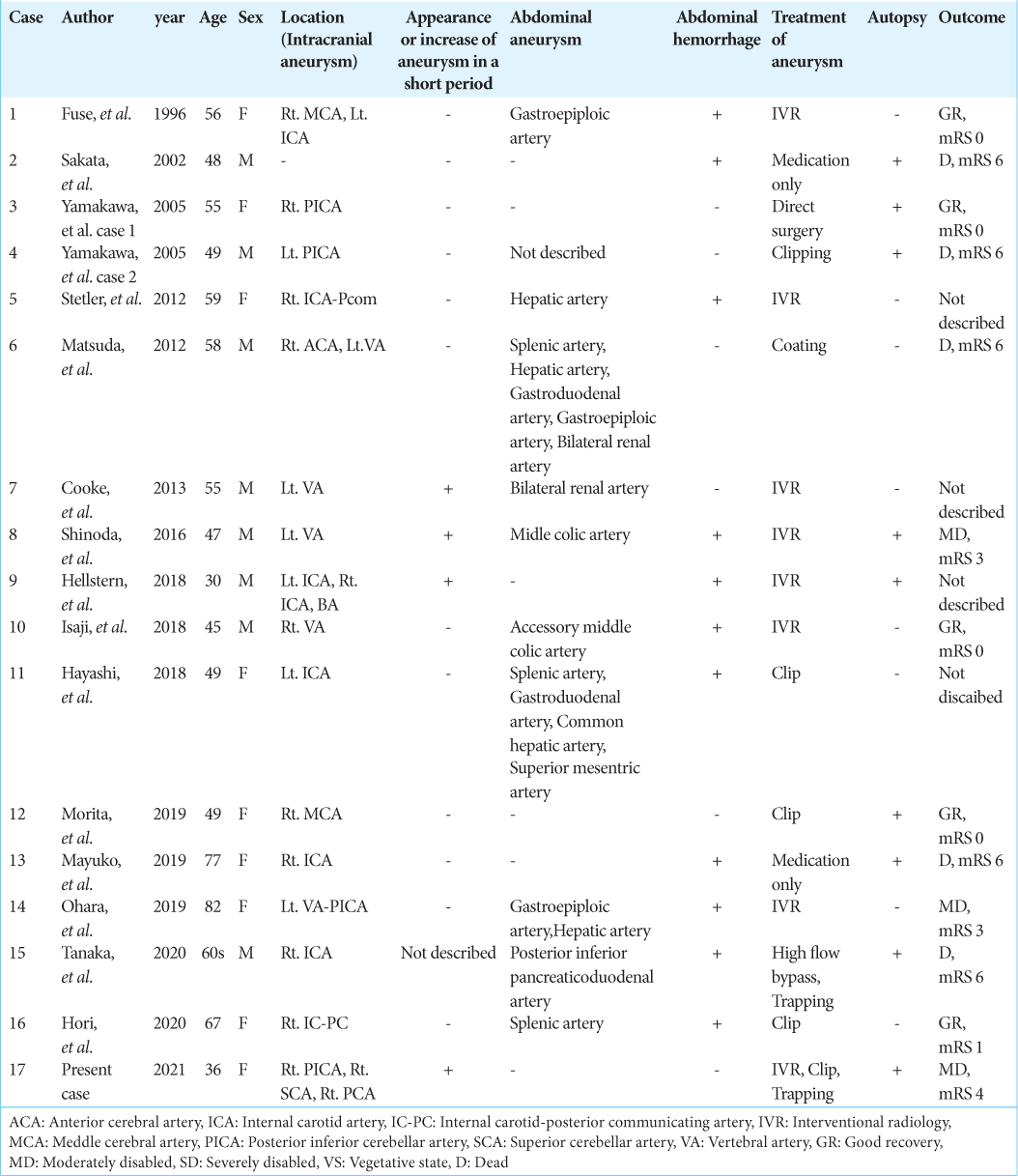
Segmental arterial mediolysis (SAM) is a pathological condition in which the arterial wall becomes vulnerable due to degeneration of the arterial tunica media, resulting in arterial dissection and aneurysm formation. SAM is a pathological condition proposed by Salvin et al. in 1976 and was the first vascular lesion reported by Inayama et al. in 1992 in Japan. SAM is often reported as the cause of intra-abdominal hemorrhage due to a ruptured abdominal aneurysm. However, there are also reports of complications of subarachnoid hemorrhage (SAH) and intra-abdominal hemorrhage, and rarely, intracranial blood vessels alone.
In this report, we present a case of multiple cerebral aneurysms forming only in intracranial blood vessels, followed by an enlargement, a rupture, or disappearance of aneurysms in a short period. Pathological findings of some of the aneurysms resulted in a definitive diagnosis of SAM.
CASE REPORT
Clinical history
Case: A 36-year-old female Medical history: Migraine Clinical findings: Japan Coma Scale (JCS) score of I-1 and Glasgow Coma Scale score of 13 (E3V4M6). No neurological deficits were observed History: A pregnant woman at 35 weeks’ gestation was referred to our department because of increasing headache and disturbance of consciousness.
Imaging findings
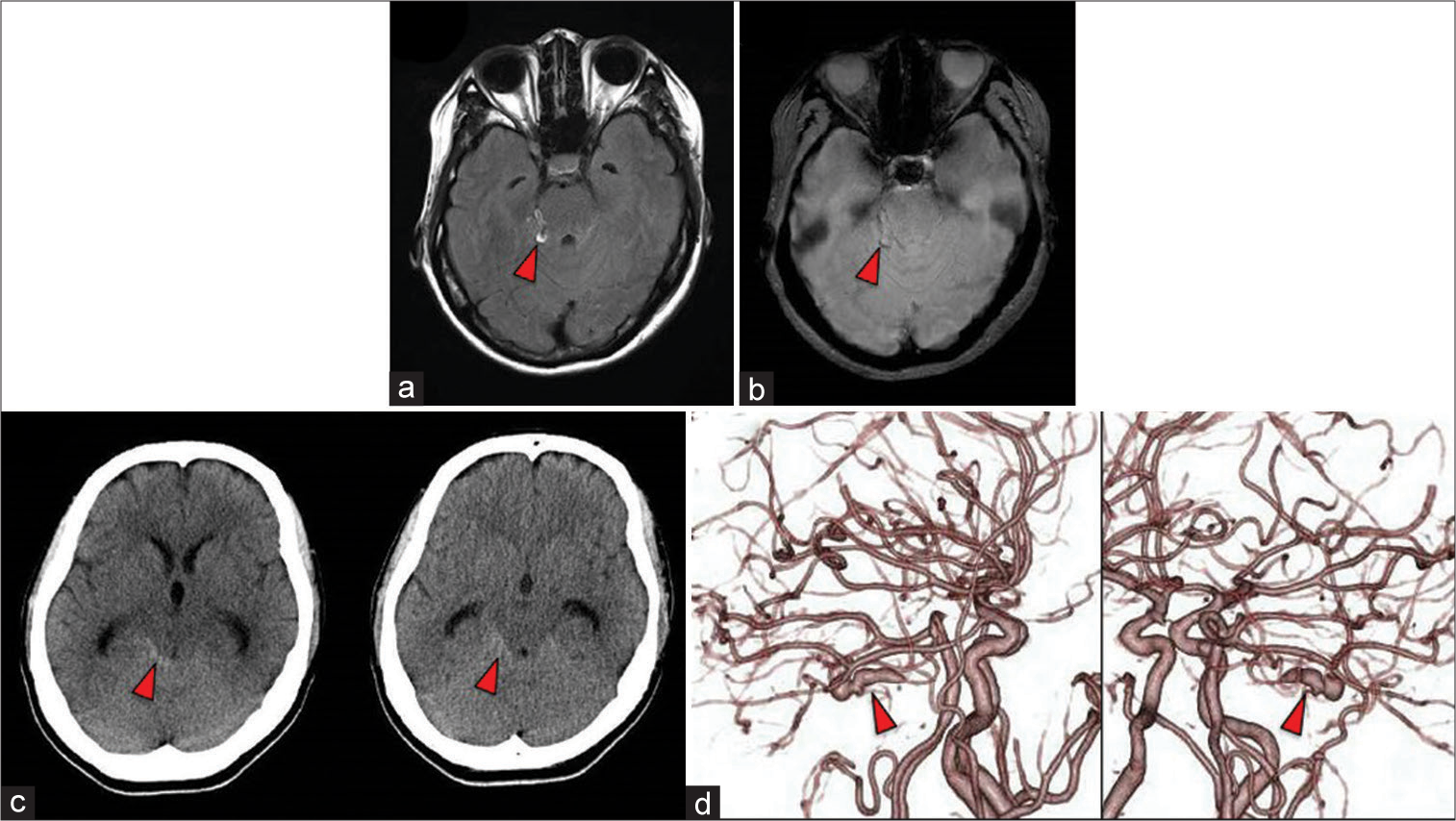
Since the patient was pregnant, magnetic resonance imaging (MRI) was given priority, considering radiation exposure. A SAH was suspected because low signal intensity on the right ambient cistern on T2 star-weighted images and high signal intensity on fluid-attenuated inversion recovery images were observed [
Figure 1:
(a) Fluid-attenuated inversion recovery image demonstrates hemorrhage in the right ambient cistern (red arrow head). (b) T2 star-weighted MR image demonstrates hemorrhage in the right ambient cistern (red arrow head). (c) Axial CT image demonstrates hemorrhage in the right ambient cistern (red arrow head). (d) CT angiography shows a dissecting cerebral aneurysm at the right distal SCA (red arrow head).
Progress (1)
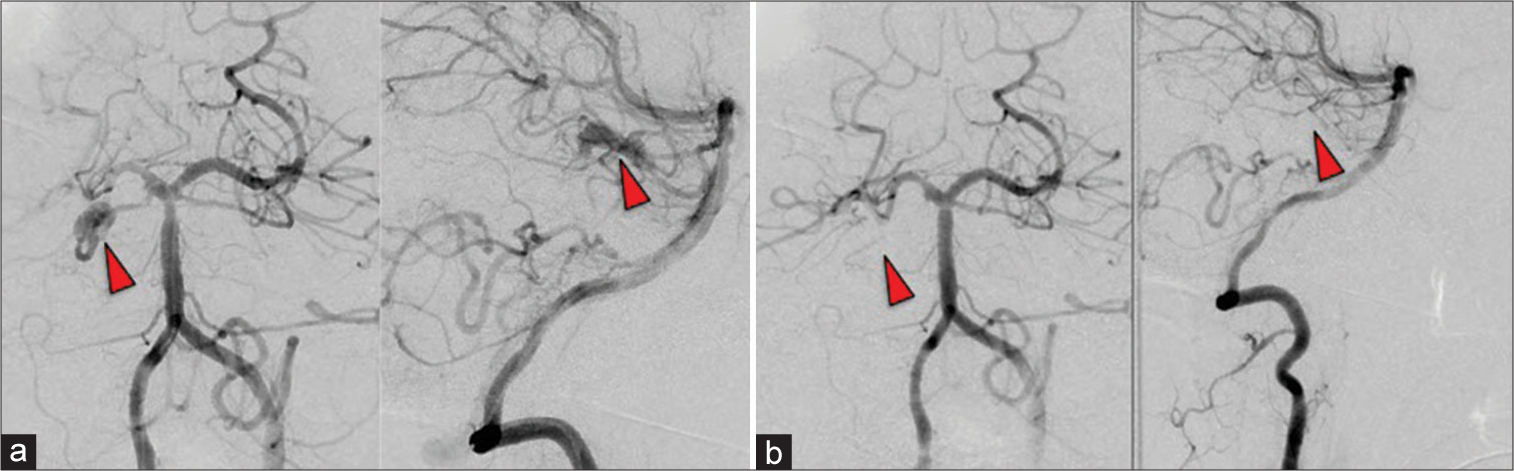
In consultation with the obstetrics and gynecology department, it was decided to treat the aneurysm after delivery of the baby by cesarean section on day 2. The aneurysm was a deep lesion, requiring bypass and wrapping for craniotomy, which was considered highly risky. Therefore, endovascular treatment was performed. Embolization of the aneurysm from the main trunk of the SCA was performed using n-butyl cyanoacrylate since it was difficult to guide the catheter to the aneurysm site [
Progress (2)
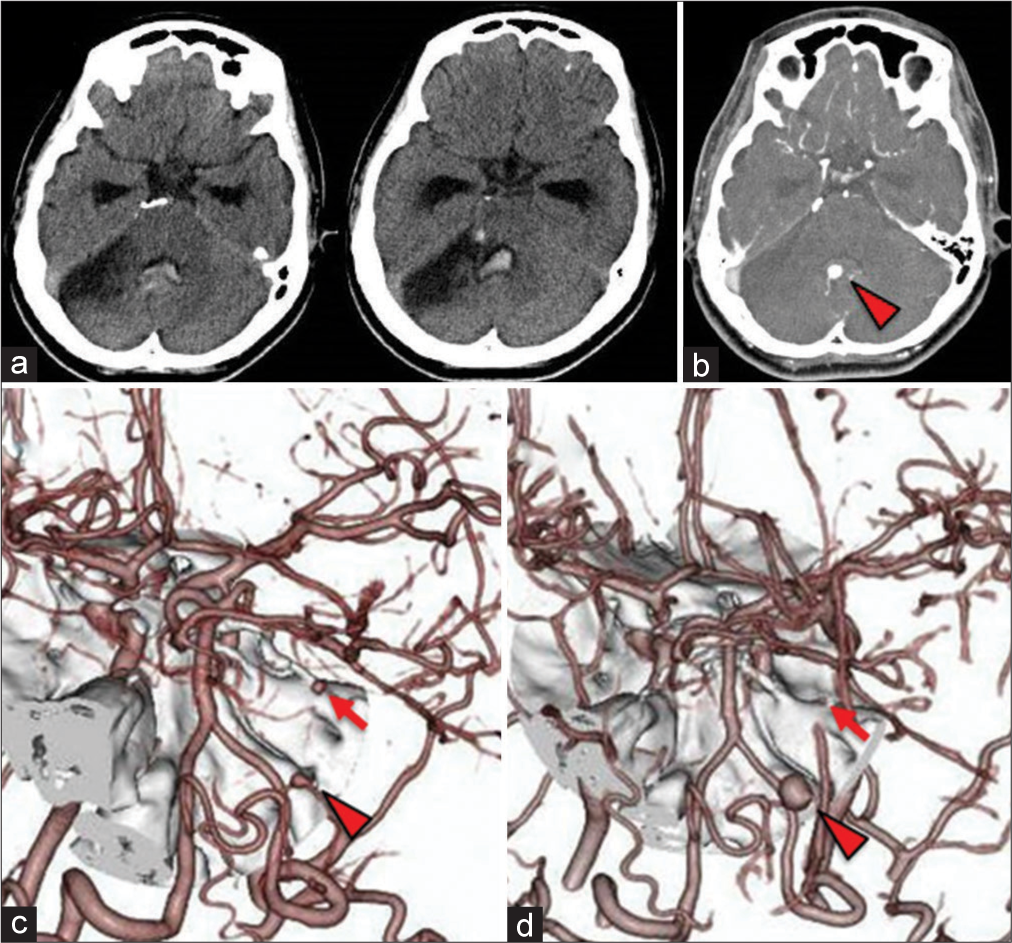
Ten weeks after the transfer, the patient developed sudden headache during rehabilitation. Head CT showed bleeding in the fourth ventricle and she was readmitted to the hospital. The 3D-CT angiography showed an enlargement of the previously observed aneurysm in the periphery of the right PICA, which was considered to be the bleeding source because it coincided with the bleeding site. Other aneurysms also showed changes in a short period (e.g., the disappearance of the peripheral aneurysm) [
Surgical findings
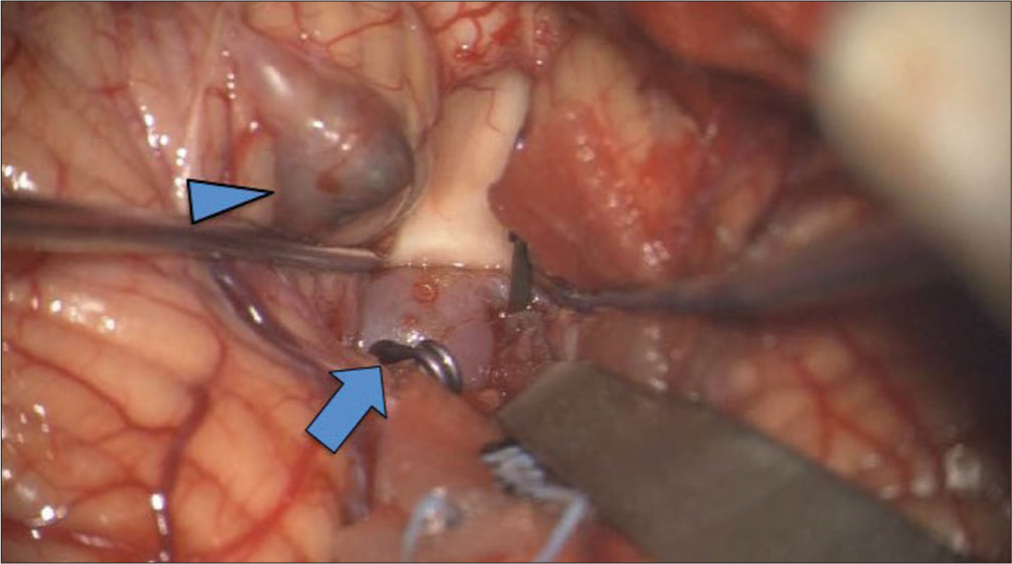
Direct surgery was performed for the peripheral right PICA aneurysm. Bilateral occipital subcranial craniotomy was performed in the prone position, the right PICA was traced to the periphery, and the aneurysm in the fourth ventricle was trapped and removed with the telovelotonsillar segment [
Figure 4:
Intraoperative findings show an aneurysm in the fourth ventricle in addition to the right distal PICA aneurysm (blue arrow). Both were trapped and removed. A thrombotic unruptured aneurysm on the right cerebellar hemisphere (blue arrow head) that was not visualized by 3D-CT was also removed.
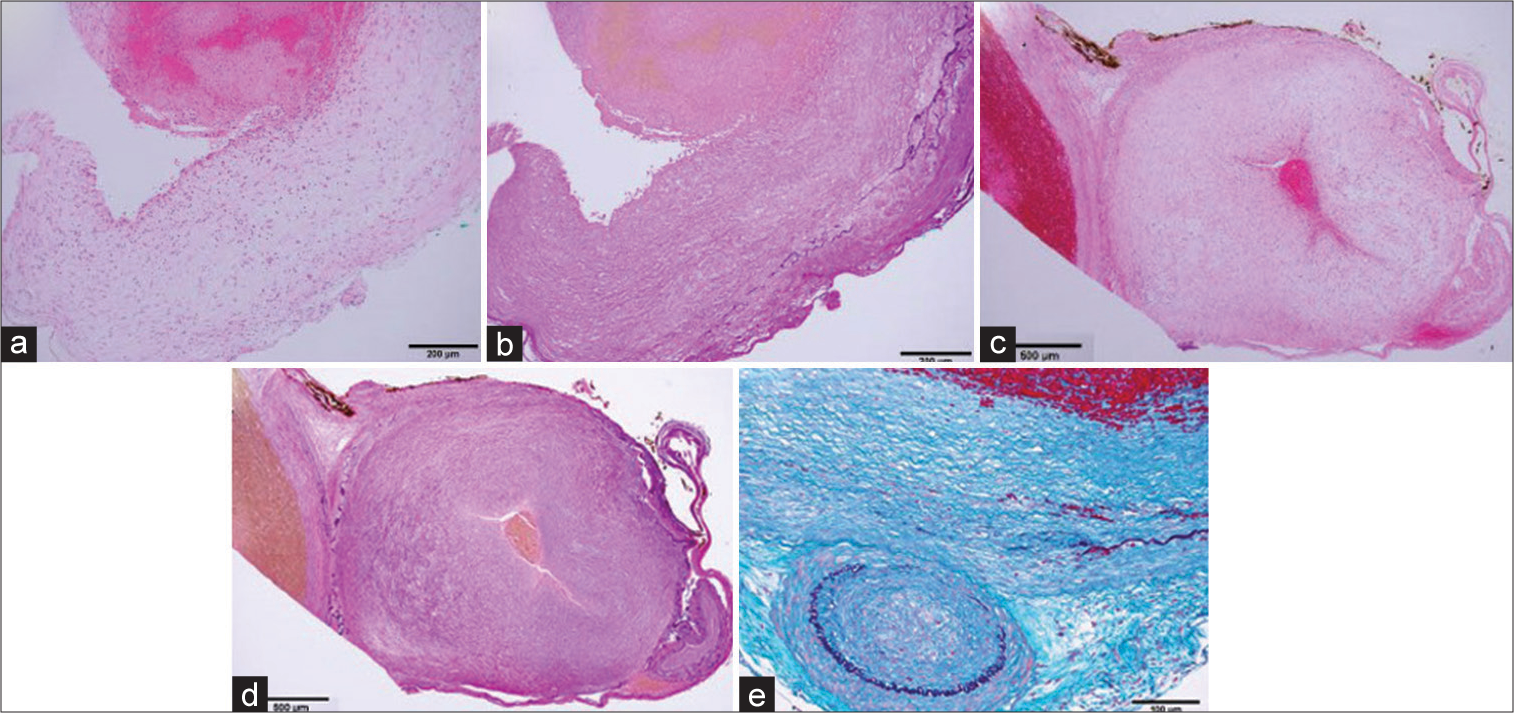
Histopathological findings
Histopathological examination by Elastica van Gieson staining revealed that the internal elastic lamina was torn in the ruptured aneurysm and segmentally missing even in the unruptured aneurysm. On the other hand, no calcification of the blood vessel wall or infiltration of inflammatory cells was observed, and the patient was pathologically diagnosed with SAM [
Figure 5:
In the ruptured aneurysm, the internal elastic lamina is segmentally torn. (a) Histopathological examination. (b) Elastica van Gieson. Even in an unruptured aneurysm, the internal elastic plate is segmentally missing. (c) Histopathological examination. (d) Elastica van Gieson. (e) Masson trichrome.
Postoperative course
Postoperative examination showed no obvious exacerbation of symptoms compared to preoperative examination; however, JCS I-1 and scanning speech remained. Since the pathological diagnosis was SAM, a CT scan and renal artery echo were performed to examine the abdominal cavity, but no obvious intra-abdominal aneurysm was noted. In addition, blood tests were negative for various antinuclear antibodies, and vasculitis workup was negative. She was transferred to the hospital and rehabilitation was continued.
DISCUSSION
In this case, a ruptured and an unruptured aneurysm were removed by surgery, and the pathological diagnosis led to a definitive diagnosis of SAM. As no abdominal aneurysm was found on whole-body CT, this is a very rare case that involved only the intracranial vessels.
SAM is a noninflammatory aneurysm without calcification, and a similar disease is fibromuscular dysplasia (FMD), which is a noninflammatory and nonarteriosclerotic arterial disease. The characteristic imaging findings of FMD include the string-of-beads sign;[
FMD is histopathologically characterized by thickening or thinning of the blood vessel wall due to hyperplasia or the disappearance of smooth muscle and fibers.[
There are two notable points in this case report. First, in the intracranial artery, the known unruptured aneurysm enlarged and ruptured within a short period after the dissociative aneurysm ruptured. It is considered that this is related to the pathological condition of remodeling and changes in the blood vessel wall could be confirmed by pathological examination. The other point is that the aneurysm that was not detected by preoperative 3D-CT angiography was identified intraoperatively. The reason why the aneurysm could not be identified on imaging was thought to be because the inside of the aneurysm was completely thrombosed. The unruptured aneurysm found in the periphery of the right posterior cerebral artery disappeared on the images at the time of readmission, which may be due to the thrombus in the aneurysm. In cases where SAM cannot be completely ruled out, such as multiple cerebral aneurysms with dynamic changes in imaging findings or an atypical course for arterial dissection, closer follow-up of intracranial aneurysms may be required, even after the aneurysm treatment.
In this case, endovascular treatment was chosen because of the high risk of craniotomy due to the presence of a deep aneurysm. Depending on the choice of treatment, pathological diagnosis may not be possible, as in this case, but SAM should also be considered in the differential diagnosis when dynamic changes of atypical dissecting aneurysms or multiple cerebral aneurysms are observed. If new aneurysms or the enlargement of existing aneurysms are detected, early surgery may be necessary. On the other hand, in case studies of long-term follow-up of SAM, it has been reported that once the active phase is over, aneurysms no longer form.[
CONCLUSION
In this study, we report a case with multiple coexisting aneurysms that changed in a short period after SAH due to the rupture of a dissecting aneurysm, and one of the aneurysms ruptured again. Histopathological examination confirmed SAM diagnosis. SAM is a disease that causes aneurysms to form in a short period due to vascular remodeling. When a noninflammatory aneurysm without calcification is observed on imaging, as in this case, SAM should be considered in the differential diagnosis for further investigation. Pathological diagnosis is necessary for definitive diagnosis, but it is necessary to consider detailed imaging follow-up even in the absence of pathological tissue confirmation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Brinza EK, Gornik HL. Fibromuscular dysplasia: Advances in understanding and management. Cleve Clin J Med. 2016. 83: S45-51
2. Cooke DL, Meisel KM, Kim WT, Stout CE, Halbach VV, Dowd CF. Serial angiographic appearance of segmental arterial mediolysis manifesting as vertebral, internal mammary and intra-abdominal visceral artery aneurysms in a patient presenting with subarachnoid haemorrhage and review of the literature. J Neurointerv Surg. 2013. 5: 478-82
3. Fuse T, Takagi T, Yamada K, Fukushima T. Systemic multiple aneurysms of the intracranial arteries and visceral arteries: Case report. Surg Neurol. 1996. 46: 258-61
4. Hayashi S, Hosoda K, Nishimoto Y, Nonaka M, Higuchi S, Miki T. Unexpected intraabdominal haemorrhage due to segmental arterial mediolysis following subarachnoid haemorrhage: A case of ruptured intracranial and intraabdominal aneurysms. Surg Neurol Int. 2018. 9: 175
5. Hellstern V, Perez AM, Meinecke KP, Bazner H, Ganslandt O, Henkes H. Concomitant retroperitoneal and subarachnoid haemorrhage due to segmental arterial Mediolysis: Case report and review of the literature. Clin Neuroradiol. 2018. 28: 445-50
6. Hori E, Shibata T, Okamoto S, Kubo M, Horie Y, Kuroda S. An intraabdominal haemorrhage affected by catecholamine surges caused by subarachnoid haemorrhage: A case report. Neurol Surg. 2020. 48: 835-40
7. Inazuka M, Imazato D, Yamazaki K, Maegawa T, Takahashi Y, Kikuchi A. A case of segmental arterial mediolysis: Subarachnoid haemorrhage followed by abdominal bleeding. Neurol Surg. 2019. 47: 543-50
8. Isaji T, Ohshima T, Miyachi S, Matsuo N, Kawaguchi R, Takayasu M. Treatment of ruptured vertebral artery dissection and abdominal haemorrhage associated with segmental arterial mediolysis using endovascular coil embolization. World Neurosurg. 2018. 116: 44-9
9. Iwanaga A, Tamehiro K, Matsuura Y, Kimura Y, Higaki K, Saruwatari A. Treatment of segmental arterial mediolysis. Jpn J Gastroenterol Surg. 2019. 52: 345-57
10. Ko M, Kamimura K, Ogawa K, Tominaga K, Sakamaki A, Kamimura H. Diagnosis and management of fibromuscular dysplasia and segmental arterial mediolysis in gastroenterology field: A mini-review. World J Gastroenterol. 2018. 24: 3637-49
11. Ko M, Kamimura K, Sakamaki A, Niwa Y, Tominaga K, Mizuno K. Rare mesenteric arterial diseases: Fibromuscular dysplasia and segmental arterial mediolysis and literature review. Intern Med. 2019. 58: 3393-400
12. Matsuda R, Hironaka Y, Takeshima Y, Park YS, Nakase H. Subarachnoid haemorrhage in a case of segmental arterial mediolysis with coexisting intracranial and intraabdominal aneurysms. J Neurosurg. 2012. 116: 948-51
13. Morita T, Maki Y, Notohara K, Kinosada M, Yasuda T, Chin M. Subarachnoid haemorrhage from a distal middle cerebral artery aneurysm possibly related to segmental arterial mediolysis. World Neurosurg. 2019. 122: 429-32
14. Nishikawa Y, Hoshina K, Sasaki H, Hosaka A, Yamamoto K, Okamoto H. Acute remodeling of an adjoining aneurysm after endovascular treatment of a ruptured splanchnic arterial aneurysm: A case of clinically diagnosed segmental arterial Mediolysis. Ann Vasc Dis. 2012. 5: 449-53
15. Ohara J, Yamao Y, Ishii A, Shimizu H, Kikuchi T, Takenobu Y. Possible segmental arterial mediolysis associated with intraperitoneal haemorrhage in the acute stage of subarachnoid haemorrhage: A case report. Neurol Surg. 2019. 47: 97-103
16. Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, Gray BH. The United States registry for fibromuscular dysplasia: Results in the first 447 patients. Circulation. 2012. 125: 3182-90
17. Sakata N, Takebayashi S, Shimizu K, Kojima M, Masawa N, Suzuki K. A case of segmental mediolytic arteriopathy involving both intracranial and intraabdominal arteries. Pathol Res Pract. 2002. 198: 493-7
18. Sethi A, Hyde JR, Thaler CM, Skeik N. Extensive cerebrovascular and visceral artery dissection and pseudoaneurysm with underlying segmental arterial mediolysis. Ann Vasc Surg. 2017. 44: 422.e9-13
19. Shinoda N, Hirai O, Mikami K, Bando T, Shimo D, Kuroyama T. Segmental arterial mediolysis involving both vertebral and middle colic arteries leading to subarachnoid and intraperitoneal haemorrhage. World Neurosurg. 2016. 88: 694.e5-10
20. Stetler WR, Pandey AS, Mashour GA. Intracranial aneurysm with concomitant rupture of an undiagnosed visceral artery aneurysm. Neurocrit Care. 2012. 16: 154-7
21. Tanaka K, Fujiwara M, Okuda Y, Ishida F, Suzuki H. A ruptured blood blister-like aneurysm associated with intraperitoneal haemorrhage due to segmental arterial mediolysis: A case report and literature review. World Neurosurg. 2020. 134: 79-85
22. Yamakawa H, Kaku Y, Yoshimura S, Ohkuma A, Sakai N. Two cases of dissecting aneurysm of the distal posterior inferior cerebellar artery: Possible involvement of segmental mediolytic arteriopathy in the pathogenesis. Clin Neurol Neurosurg. 2005. 107: 117-22
23. Yoshida S, Eguchi K, Onodera K, Suzuki K, Fujishiro K, Riku S. Bilateral internal carotid artery occlusion and severe basilar artery stenosis in a patient with fibromuscular dysplasia: A case report. Clin Neurol. 2013. 53: 439-45