- Department of Neurosurgery, Saiseikai Toyama Hospital, Toyama, Japan
- Department of Neurosurgery, Tonami General Hospital, Toyama, Japan.
Correspondence Address:
Tsuyoshi Tsukada, Department of Neurosurgery, Saiseikai Toyama Hospital, Toyama, Japan.
DOI:10.25259/SNI_612_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tsuyoshi Tsukada1, Toru Masuoka2, Michiya Kubo1. A case of temporary occlusion of donor artery after secondary generalized seizure in a patient with superficial temporal artery-middle cerebral artery bypass. 15-Sep-2023;14:330
How to cite this URL: Tsuyoshi Tsukada1, Toru Masuoka2, Michiya Kubo1. A case of temporary occlusion of donor artery after secondary generalized seizure in a patient with superficial temporal artery-middle cerebral artery bypass. 15-Sep-2023;14:330. Available from: https://surgicalneurologyint.com/surgicalint-articles/12557/
Abstract
Background: To prevent stroke recurrence, a superficial temporal artery-middle cerebral artery (STA–MCA) bypass for atherosclerotic cerebrovascular occlusive disease is performed. Post stroke epilepsy is known as serious sequelae of stroke. Herein, we present a case of a 60-year-old man who underwent STA–MCA bypass for the prevention of stroke recurrence; however, the donor artery was deemed to be temporally occluded secondary to generalized seizure.
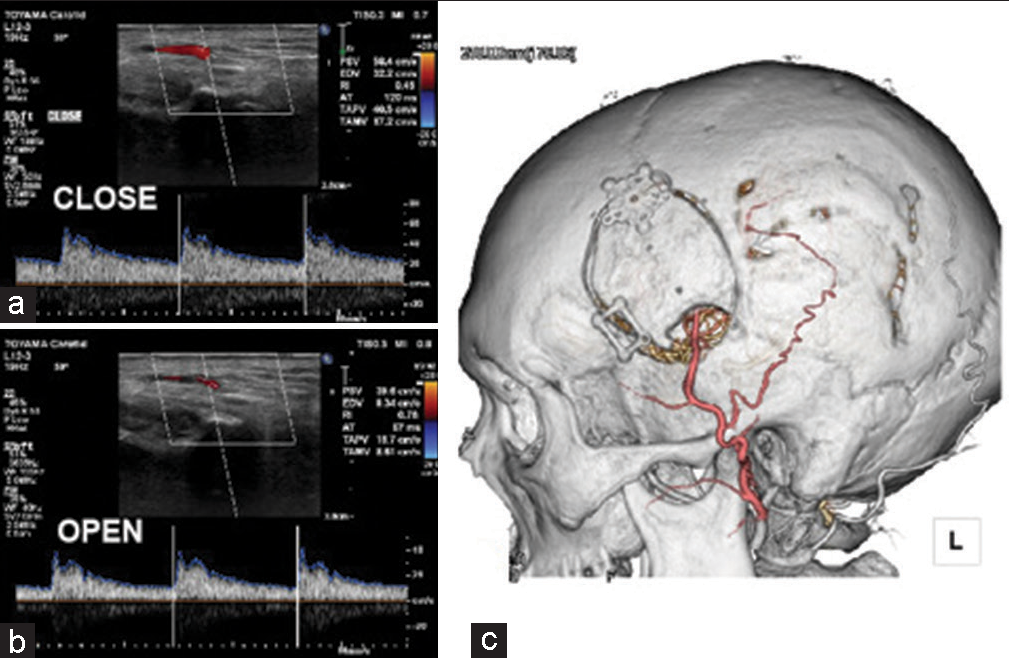
Case Description: A 60-year-old man was referred to our hospital with a diagnosis of the left cervical internal carotid artery occlusion presenting with mild aphasia and right hemiparesis. He underwent STA–MCA bypass to prevent the recurrence of stroke 1 month after the onset of symptoms. On postoperative day 7, patency of the donor artery was confirmed on magnetic resonance imaging (MRI), and no complications were noted. However, on postoperative day 14, he presented with a secondary generalized seizure. MRI was immediately performed and the donor artery was not patent with no new lesions. Several hours thereafter, the blood flow of the donor artery was confirmed using pulse Doppler; however, during mouth opening, the flow of the donor artery decreased. Computed tomography-angiography confirmed donor artery patency. An encephalogram was conducted and revealed a focal epilepsy which was compatible with stroke on MRI.
Conclusion: Post stroke epilepsy caused an unintended and forced mouth opening which led to a temporary occlusion of the donor artery after STA–MCA bypass. Thus, this complication should be recognized, and seizures should be prevented through the administration of prophylactic anti-seizure medication based on risk stratification assessment of post stroke epilepsy.
Keywords: Open mouth, Post stroke epilepsy, Secondary generalized seizure, Superficial temporal artery-middle cerebral artery bypass
INTRODUCTION
In Japan, superficial temporal artery-middle cerebral artery (STA–MCA) bypass for atherosclerotic cerebrovascular occlusive disease is currently performed to prevent stroke recurrence. Specifically, patients with severe hemodynamic impairment who demonstrate cerebral blood flow <80% at rest and cerebral vascular reserve <10% are considered to be at high risk of stroke recurrence.[
CASE PRESENTATION
A 60-year-old man who is a smoker with a medical history of hypertension, hyperlipidemia, and diabetic mellitus was transferred to our hospital with a diagnosis of acute ischemic stroke secondary to a left cervical internal carotid artery occlusion. One of his pertinent medical histories was a left-sided depressed fracture in which he underwent reduction surgery during childhood. Three days before admission, he complained of mild aphasia, dysarthria, and right hemiparesis. Magnetic resonance imaging (MRI) and computed tomography (CT) angiography showed occlusion of the proximal left cervical carotid artery and ipsilateral ischemic stroke [
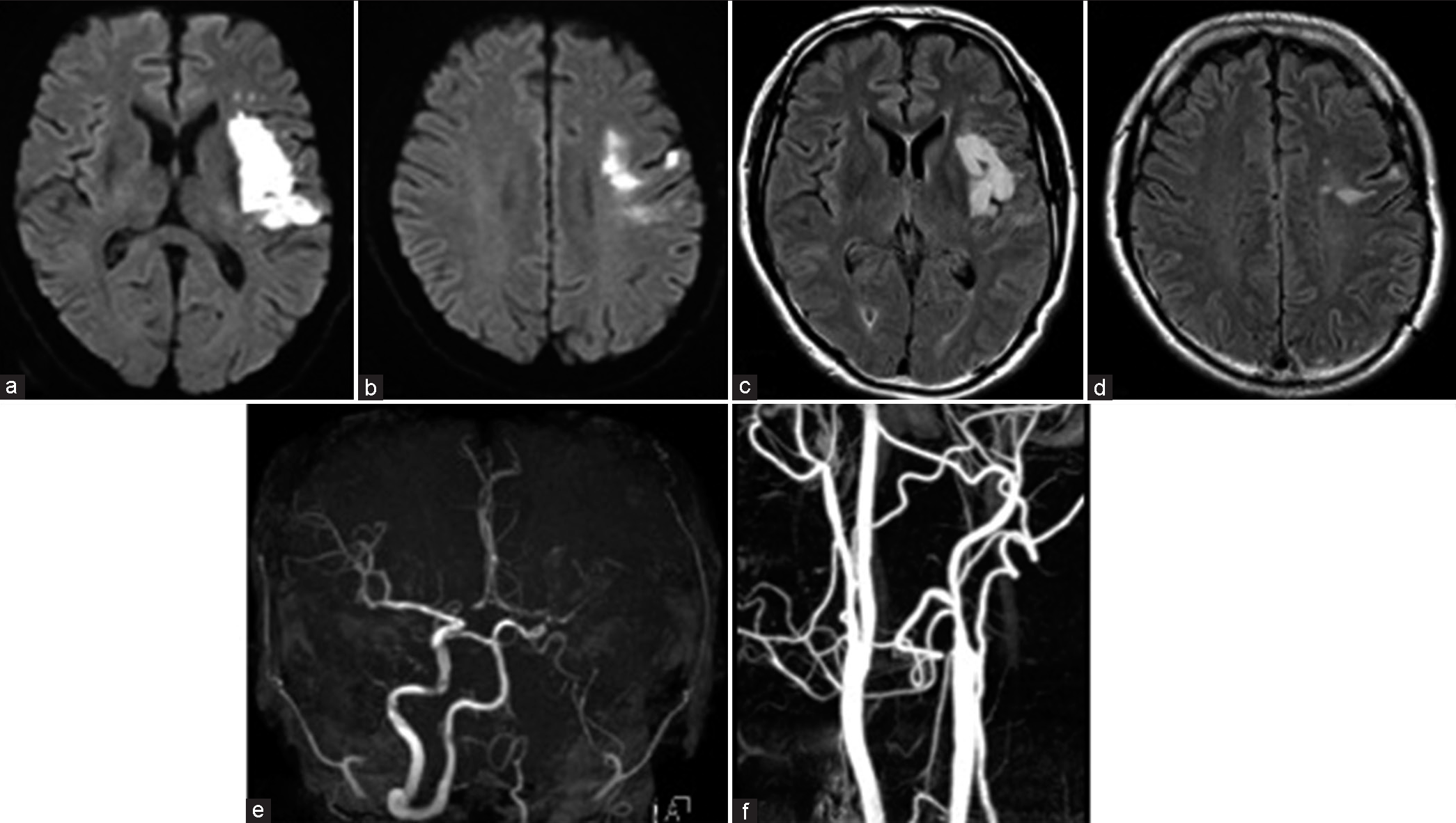
Figure 1:
(a and b) Magnetic resonance imaging (MRI) diffusion-weighted imaging (DWI) demonstrating an infarct involving the left basal ganglia, insula, and opercular region. (c and d) MRI fluid-attenuated inversion recovery showing an infarct compatible with that of DWI. (e and f) Magnetic resonance angiography reveals no flow signal intensity in the left neck internal carotid artery (ICA) and contrast computed tomography demonstrates occlusion of the left neck ICA.
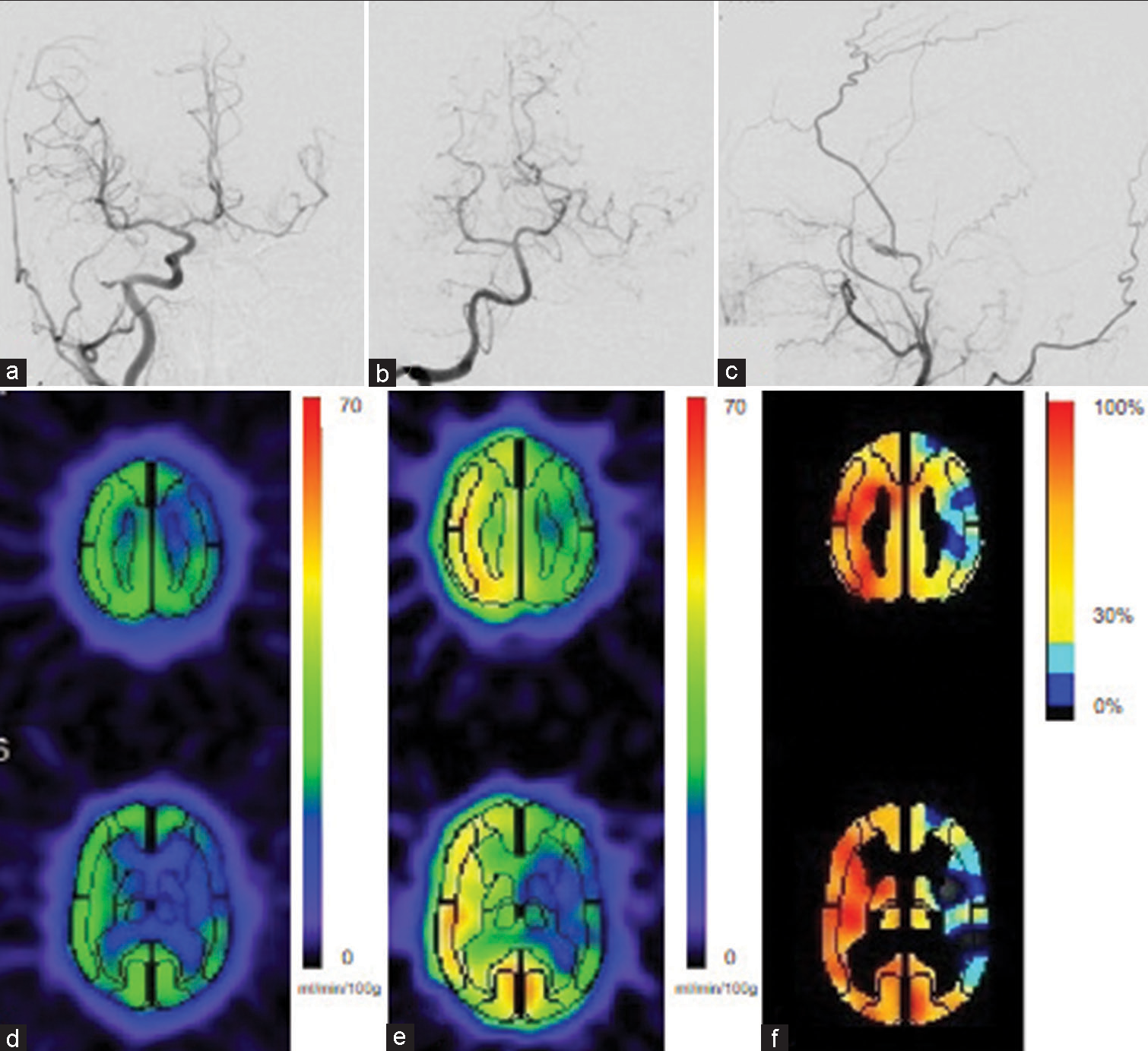
Figure 2:
(a and b) Cerebral angiography demonstrating collateral flow to the left hemisphere through the anterior communicating artery and leptomeningeal anastomoses from the posterior cerebral artery. (c) Left external carotid artery angiography showing opacification of the internal carotid artery and ophthalmic artery through the middle meningeal artery. (d-f) 123I-iodoamphetamine single– photon emission computed tomography demonstrating cerebral blood flow reduction at rest and cerebral vascular reserve in response to acetazolamide in the left hemisphere.
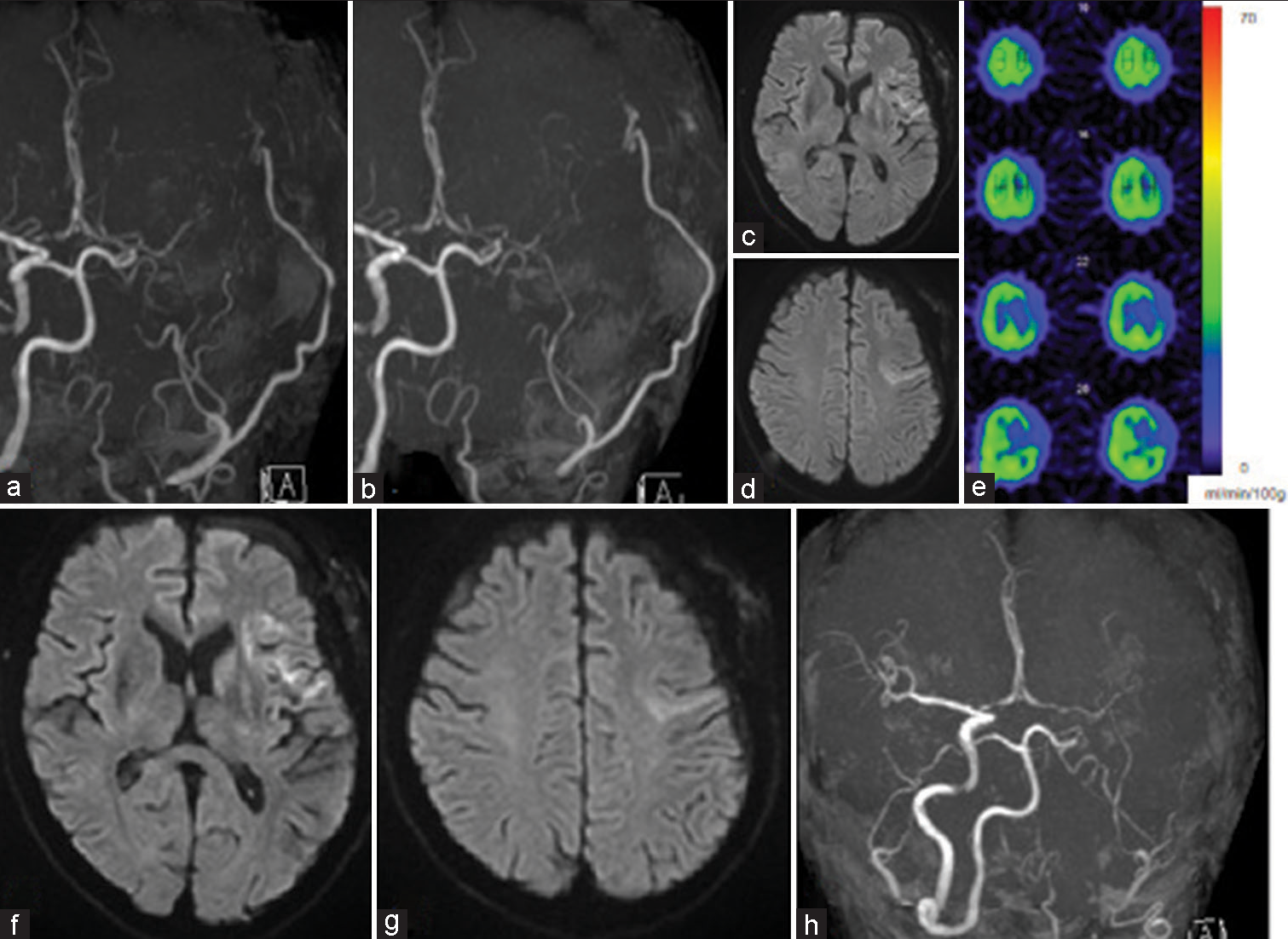
Figure 3:
(a and b) Intracranial magnetic resonance angiography (MRA) showing patency of the donor artery following a day and 7 days after superficial temporal artery-middle cerebral artery bypass, respectively. (c and d) Magnetic resonance imaging (MRI) diffusion-weighted imaging (DWI) revealed no new infarction on postoperative day 7. (e) 123I-iodoamphetamine single-photon emission computed tomography showed no apparent region of hyperperfusion on postoperative day 9. (f-h) Immediate MRI after secondary generalized seizure demonstrated no new infarction on DWI and MRA revealing diminishing signal intensity of the donor artery.
DISCUSSION
STA–MCA bypass is currently performed in Japan for atherosclerotic cerebrovascular disease, moyamoya disease, and complex aneurysm surgery, among others. Although some complications have been described following STA– MCA bypass, this procedure is considered relatively safe.[
CONCLUSION
Secondary generalized seizure following stroke causes unintended and forced mouth opening, which leads to the temporary occlusion of a donor artery after STA–MCA bypass. The serious complication of STA–MCA bypass should be recognized, and seizures should be prevented through the prophylactic administration of anti-seizure medication based on risk stratification assessment of post stroke epilepsy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abe H, Katsuta T, Miki K, Higashi T, Inoue T. Temporary steno-occlusive change in the donor artery during mouth opening (big bite ischemic phenomenon) after superficial temporal artery to middle cerebral artery bypass in adult patients with moyamoya disease and atherosclerosis. Acta Neurochir Suppl. 2016. 123: 123-8
2. Chida K, Ogasawara K. Systematic review of complication for proper informed consent (11) STA-MCA bypass surgery. No Shinkei Geka. 2013. 41: 1111-8
3. DeToledo JC, Ramsay RE. Patterns of involvement of facial muscles during epileptic and nonepileptic events: Review of 654 events. Neurology. 1996. 47: 621-5
4. Farrell JS, Gaxiola-Valdez I, Wolff MD, David LS, Dika HI, Geeraert BL. Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX-2 dependent. Elife. 2016. 5: e19352
5. Ferreira-Atuesta C, Döhler N, Erdélyi-Canavese B, Felbecker A, Siebel P, Scherrer N. Seizures after ischemic stroke: A matched multicenter study. Ann Neurol. 2021. 90: 808-20
6. Galovic M, Döhler N, Erdélyi-Canavese B, Felbecker A, Siebel P, Conrad J. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): A multivariable prediction model development and validation study. Lancet Neurol. 2018. 17: 143-52
7. Hishikawa T, Date I. Evidence of efficacy of superficial temporal artery-middle cerebral artery bypass in Japan. No Shinkei Geka. 2022. 50: 745-51
8. Horie N, Okazaki T. Indications for extracranial-intracranial bypass surgery. No Shinkei Geka. 2022. 50: 727-34
9. Kataoka H, Miyamoto S, Ogasawara K, Iihara K, Takahashi JC, Nakagawara J. Results of a prospective cohort study on symptomatic cerebrovascular occlusive disease showing mild hemodynamic compromise [Japanese extracranial-intracranial bypass trial (JET)-2 study]. Neurol Med Chir (Tokyo). 2015. 55: 460-8
10. Katsuta T, Abe H, Miki K, Inoue T. Reversible occlusion of donor vessel caused by mouth opening after superficial temporal artery-middle cerebral artery anastomosis in adult moyamoya patients. J Neurosurg. 2015. 123: 670-5
11. Miyamoto S, Ogasawara K, Kuroda S, Itabashi R, Toyoda K, Itoh Y. Japan stroke society guideline 2021 for the treatment of stroke. Int J Stroke. 2022. 17: 1039-49
12. Newell DW. Superficial temporal artery to middle cerebral artery bypass. Skull Base. 2005. 15: 133-41
13. Yasaka M, Omae T, Tsuchiya T, Yamaguchi T. Ultrasonic evaluation of the site of carotid axis occlusion in patients with acute cardioembolic stroke. Stroke. 1992. 23: 420-2