- Department of Neurological Surgery, Keio University Hospital, Tokyo, Japan
- Department of Pathology, Keio University Hospital, Tokyo, Japan
Correspondence Address:
Nobuhiko Arai
Department of Neurological Surgery, Keio University Hospital, Tokyo, Japan
DOI:10.4103/sni.sni_484_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nobuhiko Arai, Satoshi Takahashi, Hatano Mami, Yukina Tokuda, Kazunari Yoshida. A case report of surgical management of hemangiopericytoma at the foramen magnum. 18-Jul-2017;8:151
How to cite this URL: Nobuhiko Arai, Satoshi Takahashi, Hatano Mami, Yukina Tokuda, Kazunari Yoshida. A case report of surgical management of hemangiopericytoma at the foramen magnum. 18-Jul-2017;8:151. Available from: http://surgicalneurologyint.com/surgicalint-articles/a-case-report-of-surgical-management-of-hemangiopericytoma-at-the-foramen-magnum/
Abstract
Background:Hemangiopericytoma (HPC) is a highly vascularized mesenchymal tumor known for its high rates of recurrence and metastasis. The extent of tumor removal is known to be the most trustful prognostic factor. Skull base HPCs are challenging to treat because of the difficulty of the surgical approach and proximity to vital vascular and neuronal structures. We successfully treated a case of HPC at the ventral foramen magnum through surgical gross tumor removal via a far-lateral transcondylar approach.
Case Description:A 38-year-old male complained of neck pain and bilateral paresthesia of his shoulders for 2 months, for which he was referred to our hospital. A magnetic resonance image (MRI) showed a 20 mm diameter mass at the ventral foramen magnum, which compressed his medulla oblongata. The tumor was gross totally removed via a far-lateral transcondylar approach. During the surgery, marked bleeding disturbed the surgical field until the main feeding artery from the direction of the dura mater was coagulated and cut. A relatively wide surgical field and a transcondylar approach were helpful to control the bleeding. The pathological examination revealed the tumor to be a HPC. After an uneventful recovery period of 9 days, the patient was discharged without neurological sequelae.
Conclusion:We successfully and completely removed an HPC near the foramen magnum, employing a wide surgical field and a transcondylar approach to help control bleeding. When the tumor is suspected preoperatively to be a hemangiocytoma or vascular-rich tumor, a surgical approach that can secure a wide surgical field should be selected.
Keywords: Far-lateral approach, foramen magnum, hemangiopericytoma, transcondylar approach
INTRODUCTION
Hemangiopericytomas (HPCs) are highly vascularized mesenchymal tumors that derive from the pericytes forming the walls of capillaries and postcapillary venules. They are relatively rare intracranial tumors with a reported incidence of 0.4% of all primary central nervous system (CNS) tumors.[
CASE REPORT
A 38-year-old man was referred to our institution with chief complaints of bilateral sensory disturbance of the hands, intermittent headache, and nausea that persisted for 2 months. He had no apparent past medical history nor familial medical history.
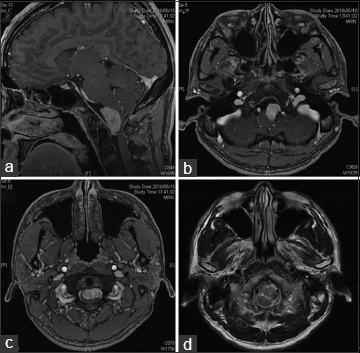
On admission, neurological examination revealed sensory disturbance of the bilateral upper extremities. T1-weighted magnetic resonance imaging (MRI) with gadolinium enhancement revealed a well-demarcated, solid mass at the ventral foramen magnum that was compressing the patient's medulla oblongata [Figure
Figure 1
T1-weighted, gadolium-enhanced MR images; a sagittal image shows a homogenously enhanced mass at the ventral foramen magnum, whose size is around 20 mm in diameter (a). On axial image, the tumor seemed to be attached to dura mater around C2. The tumor severely compressed the ventral medulla oblongata. (b and c) The axial, T2-weighted MR image showed no medullary edema (d)
Figure 2
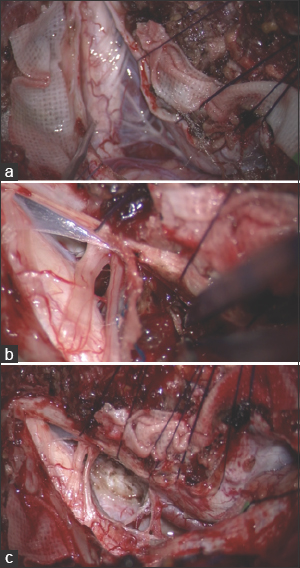
After opening the dura, the tumor, covered by the arachnoid, was identified. The tumor compressed the medulla (a). It had no severe adhesion to the brain stem or any cranial nerves. When approaching the tumor to detach it from the dura, massive hemorrhaging occurred that was difficult to control (b). After cutting the main feeder from the direction of the dura, the tumor became white and easy to manage. The whole dural attachment of the tumor was detached. Plate c shows the final view, after gross total removal of the tumor was achieved (Simpson Grade 2)
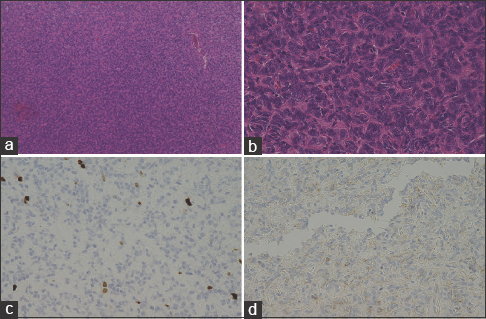
Histologically, the tumor cells had high, dense nuclear, and branching vessels inside the tumors producing a staghorn appearance [Figure
Figure 3
(a) Hematoxylin-eosin staining showing hypercellular tumor with staghorn appearance (arrow) (original magnification ×10). (b) The arrow showed mitosis. (original magnification ×40). (c) Ki-67 expressed in the HPC, nucleus positive, positive cells were less than 3% (original magnification ×40). (d) The tumor was negative for EMA staining (original magnification ×40)
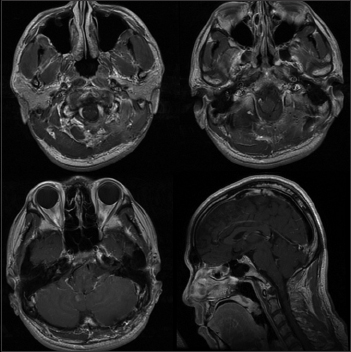
After surgery, no additional neurological sequelae appeared, and his initial symptoms of bilateral sensory disturbance of the hands completely disappeared. He was discharged on the 9th day after surgery. Follow-up MRIs were conducted periodically, and no tumor recurrence was detected at the 10-month follow-up. Radiation therapy has not been offered to the patient till now. We saved the choice of treatment in case of tumor recurrence. Postoperative MRI showed no residual or occurrence of the tumor [
DISCUSSION
Tumors at the foramen magnum are challenging to treat because of their proximity to important vascular and nervous structures. Many critical neurovascular structures in this area are sensitive to injury.[
Recently, less invasive surgery with narrow surgical view such as keyhole surgery or endoscopic surgery has been admired all around the world. In contrast, the hemorrhage in this case could not have been controlled with such a narrow corridor. Moreover, we could not have achieved gross total removal due to its poor view. This case teaches us a lesson that sufficiently wide corridor would be safe and reliable if preoperative neuroimaging showed high vascularity of tumors assumed to be challenging to remove. It is natural that to curtail the superfluous invasiveness and narrow the range of bone drilling as much as possible is very important. However, we think this inclination should not always be applied to all cases. From our experience, we aimed to create a stir in this trend and share this experience to cope with tumors case by case flexibly.
In the present case, there was massive hemorrhage during the operation while cutting the tumor. Because of the bleeding, the tumor itself and the surrounding anatomy was not fully visible. Thus, performing fine manipulation was nearly impossible until the bleeding was managed by coagulating the feeding artery. In this case, the far-lateral approach provided a large enough surgical field to find the attachment easily. After managing this, we controlled the hemorrhage and attained gross total removal.
CONCLUSION
We experienced a challenging case of HPC at the ventral foramen magnum, which was gross totally removed via a far-lateral transcondylar approach. This approach provided a significantly large surgical corridor compared to the lateral suboccipital approach, and thus allowed us to manage the tumor attachment with minimal retraction of the brainstem and relatively easy control of the bleeding, which is necessary for cases of highly vascularized tumors.
Informed consent
The patient has consented to submission of this case report to the journal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bertalanffy H, Seeger W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery. 1991. 29: 815-21
2. George B, Lot G, Boissonnet H. Meningioma of the foramen magnum: A series of 40 cases. Surg Neurol. 1997. 47: 371-9
3. Hernández-Durán S, Sánchez-Jiménez E, Pérez-Berríos J. Hemangiopericytoma of the foramen magnum in a pregnant patient: A case report and literature review. Surg Neurol Int. 2014. 28: 5-13
4. Kandenwein JA, Richter HP, Antoniadis G. Foramen magnum meningiomas—experience with the posterior suboccipital approach. Br J Neurosurg. 2009. 23: 33-9
5. Kano T, Kawase T, Horiguchi T, Yoshida K. Meningiomas of the ventral foramen magnum and lower clivus: Factors influencing surgical morbidity, the extent of tumour resection, and tumour recurrence. Acta Neurochir. 2010. 152: 79-86
6. Komotar RJ, Zacharia BE, McGovern RA, Sisti MB, Bruce JN, D’Ambrosio AL. Approaches to anterior and anterolateral foramen magnum lesions: A critical review. J Craniovertebr Junct Spine. 2010. 1: 86-99
7. Louis DN, Ohgaki H, Wiestler OD. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
8. Melone AG, D’Elia A, Santoro F, Salvati M, Delfini R, Cantore G. Intracranial hemangiopericytoma-our experience in 30 years: A series of 43 cases and review of the literature. World Neurosurg. 2014. 81: 556-62
9. Meyer FB, Ebersold MJ, Reese DF. Benign tumors of the foramen magnum. J Neurosurg. 1984. 61: 136-42
10. Miller E, Crockard HA. Transoral transclival removal of anteriorly placed meningiomas at the foramen magnum. Neurosurgery. 1987. 6: 966-8
11. Park HH, Lee KS, Hong CK. Vertebral Artery Transposition Via an Extreme-Lateral Approach for Anterior Foramen Magnum Meningioma or Craniocervical Junction Tumors. World Neurosurg. 2016. 8: 154-65
12. Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T. Intracranial hemangiopericytoma: Clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012. 118: 1628-1636
13. Samii M, Klekamp J, Carvalho G. Surgical results for meningiomas of the craniocervical junction. Neurosurgery. 1996. 39: 1086-1094
14. Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma: Long-term outcome revisited. Clinical article. J Neurosurg. 2011. 114: 747-55
15. Shidoh S, Toda M, Kawase T, Nakajima H, Tomita T, Ogawa K. Transoral vs. endoscopic endonasal approach for clival/upper cervical chordoma. Neurol Med Chir. 2014. 54: 991-8
16. Stevenson GC, Stoney RJ, Perkins RK, Adams JE. A transcervical transclival approach to the ventral surface of the brain stem for removal of a clivuschordoma. J Neurosurg. 1966. 24: 544-51
17. Suhardja A, Agur AM, Cusimano MD. Anatomical basis of approaches to foramen magnum and lower clival meningiomas: Comparison of retrosigmoid and transcondylar approaches. Neurosurg Focus. 2003. 14: e9-
18. Yamahata H, Yamaguchi S, Takayasu M, Takasaki K, Osuka K, Aoyama M. Exploitation of Simple Classification and Space Created by the Tumor for the Treatment of Foramen Magnum Meningiomas. World Neurosurg. 2016. 87: 1-7