- Department of Neurosurgery, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, United States.
- Department of Surgery, Division of Cardiothoracic Surgery, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, United States.
- Department of Pathology and Laboratory Medicine, Madison, Wisconsin, United States.
- Department of Surgery, Division of Otolaryngology-Head and Neck Surgery, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, United States.
- Department of Neurosurgery, University of Wisconsin, Madison, Wisconsin, United States.
Correspondence Address:
Amgad S. Hanna, MD, Department of Neurosurgery, University of Wisconsin, Madison, Wisconsin, United States.
DOI:10.25259/SNI_273_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jacob A. Bethel1, Daniel P. McCarthy2, Paul S. Weisman3, Tiffany A. Glazer4, Amgad S. Hanna5. A combined, supraclavicular, infraclavicular, transaxillary, and posterior subscapular approaches for en bloc resection of giant myxofibrosarcoma. 21-Apr-2023;14:144
How to cite this URL: Jacob A. Bethel1, Daniel P. McCarthy2, Paul S. Weisman3, Tiffany A. Glazer4, Amgad S. Hanna5. A combined, supraclavicular, infraclavicular, transaxillary, and posterior subscapular approaches for en bloc resection of giant myxofibrosarcoma. 21-Apr-2023;14:144. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12279
Abstract
Background: Myxofibrosarcoma (MFS) is a rare and locally infiltrative tumor that commonly occurs in extremities in older adults; however, truncal and head and neck cases have been reported. They are characterized by multinodular growth, incomplete fibrous septa, and myxoid stroma. Surgical resection is the mainstay of treatment.
Case Description: The authors report a case of a combined, supraclavicular, infraclavicular, transaxillary, and posterior subscapular approaches for resection of giant MFS.
Conclusion: The anatomical complexity and rarity of tumors involving the brachial plexus impose many challenges onto surgeons performing surgical resections. Treatment choices and surgical outcomes rely heavily on meticulous multidisciplinary planning, anatomical knowledge, careful dissection, and extent of resection. This case is unique in utilizing four different approaches to the brachial plexus to resect one tumor.
Keywords: Brachial plexus, Infraclavicular, Myxofibrosarcoma, Posterior subscapular, Soft-tissue sarcoma, Supraclavicular, Transaxillary
INTRODUCTION
Myxofibrosarcomas (MFSs) are malignant tumors of myofibroblasts. The World Health Organization defines MFS as a malignant connective tissue neoplasia of fibroblastic origin.[
CASE REPORT
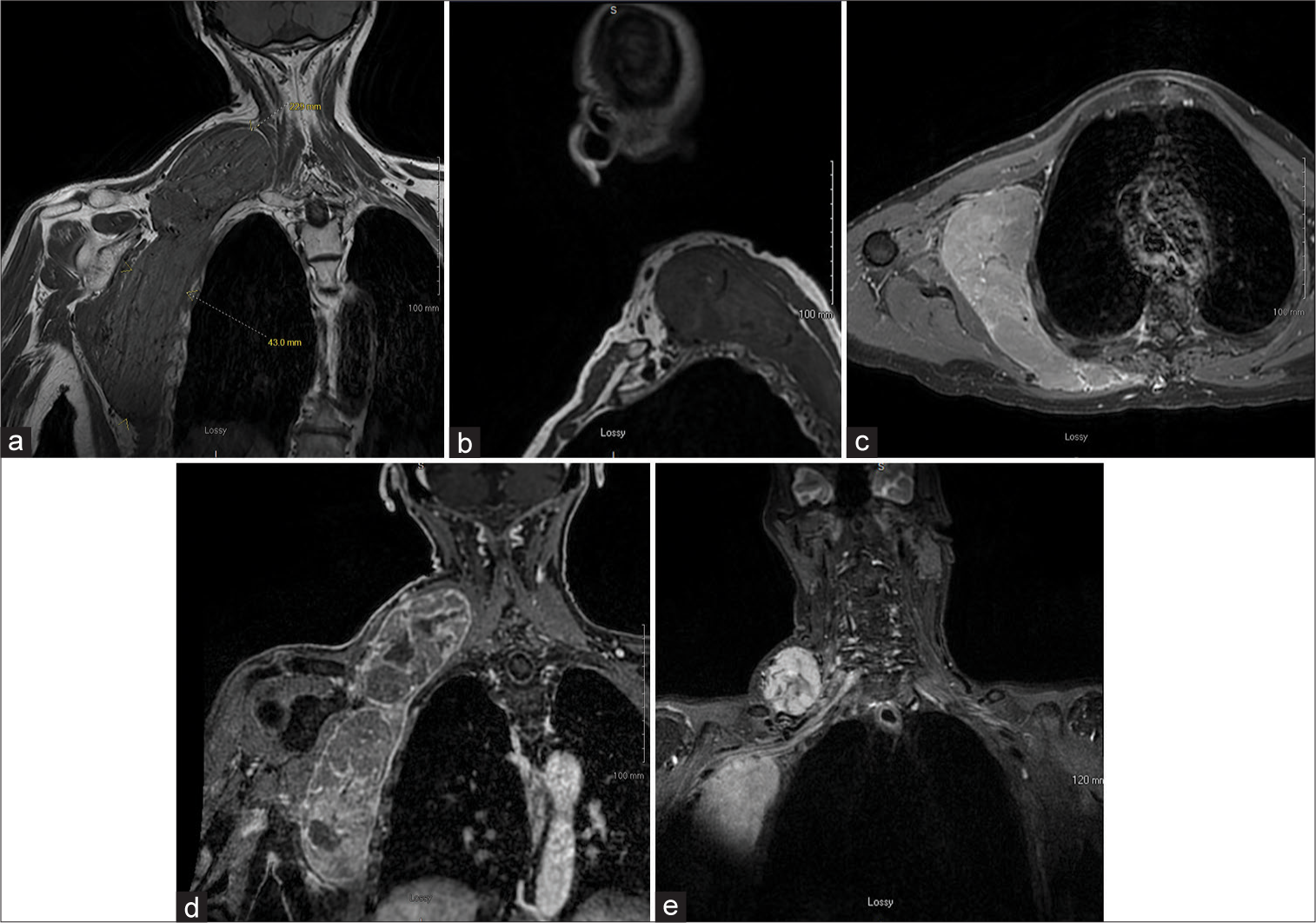
The patient was a 74-year-old former smoker with no other significant medical history who presented with a palpable mass in his right axilla and right neck. MRI of his chest was performed and demonstrated a large infiltrative solid heterogeneous mass centered in deep right lateral chest wall fat with infiltration into the right lower neck region [
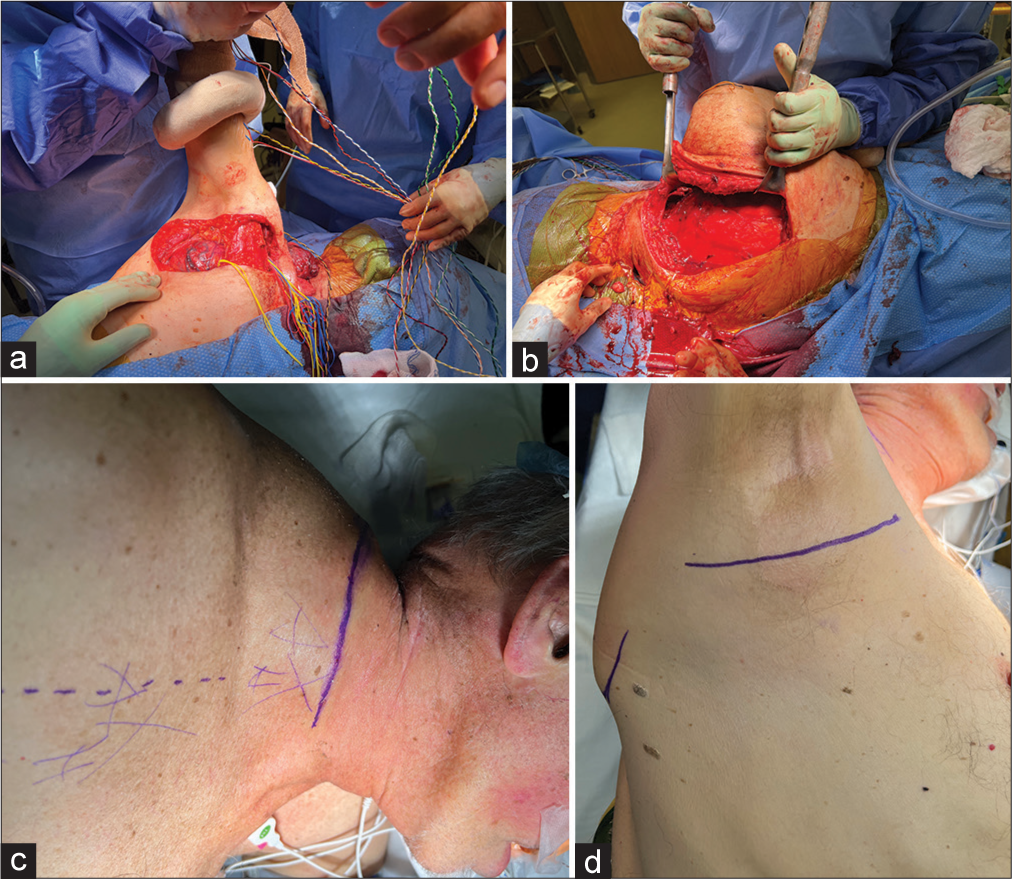
After consent was obtained, approximately 2 weeks after radiation therapy, the patient was brought to the operating room and was positioned in a left lateral decubitus manner with free mobility of the arm to facilitate various exposures during different phases of the operation [
Incisions were planned to achieve a supraclavicular, infraclavicular, transaxillary, and posterior subscapular approaches [
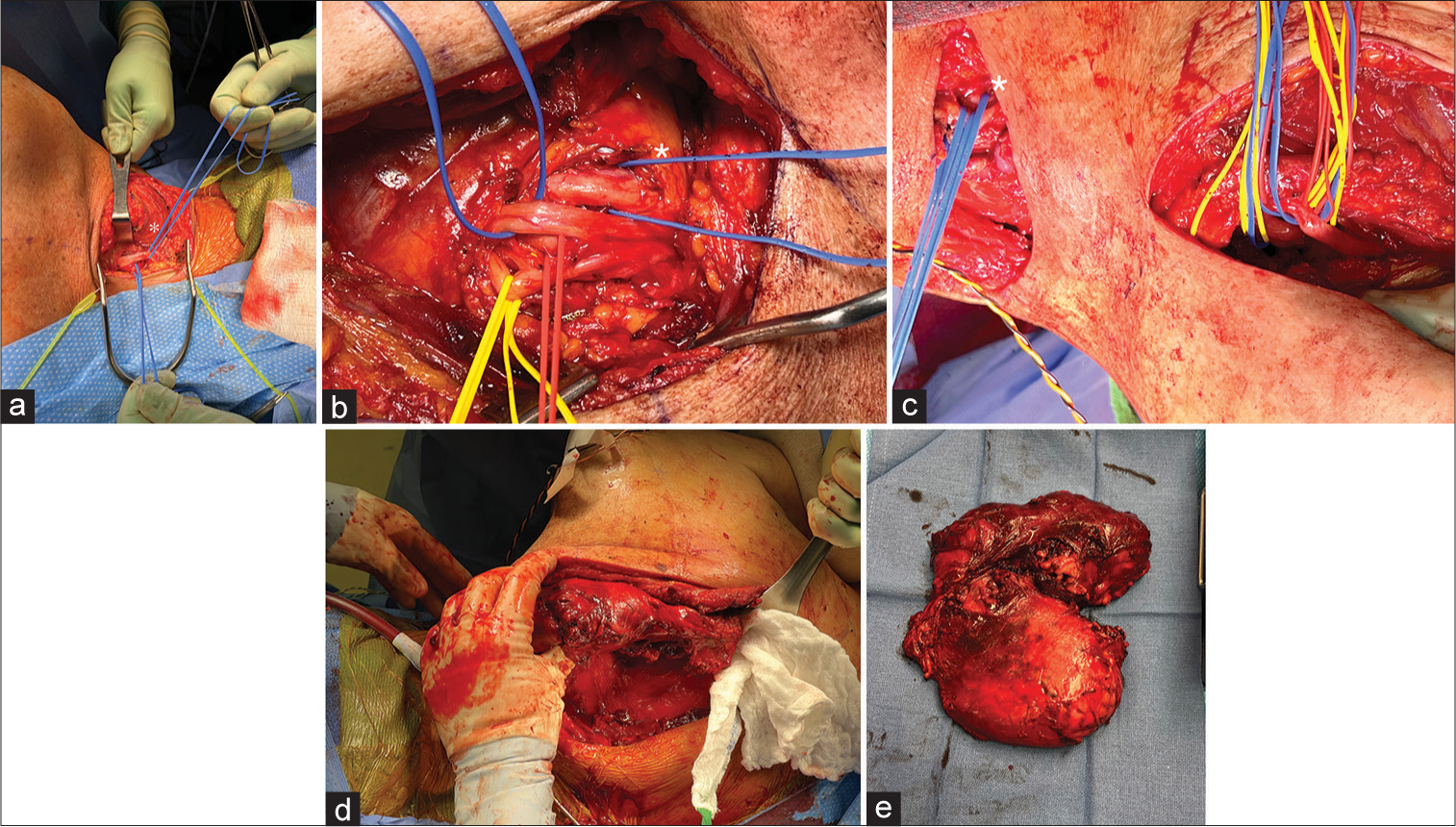
Figure 3:
(a) Supraclavicular incision with mass retracted laterally, dissection flap containing spinal accessory nerve, and upper trunk (*blue loop). (b) Infraclavicular exposure with medial cord (yellow loop) lateral cord (*blue loop) and posterior cord (blue loop), and axillary artery (red loop) with underlying mass. (c) Axillary and infraclavicular incisions with proximal and distal brachial plexus exposure including musculocutaneous nerve (*blue loop) and median nerve (blue loop). (d) Posterior subscapular incision with dissection off posterior chest wall and lateral mass retraction. (e) Pathology specimen of gross tumor.
Neurosurgery team members then identified the brachial plexus, exiting between the anterior and middle scalene muscles, and descending anterior to the mass. CN 11 was further dissected and confirmed with nerve stimulator running over the posterior superficial portion of mass. The nerve was bluntly dissected free of soft-tissue attachments connecting it to the mass. A flap was elevated containing the nerve for protection throughout remainder of procedure, the nerve was not skeletonized to avoid excessive traction. At this point, we began dissecting the mass from surrounding soft-tissue attachments circumferentially using combination of LigaSure™ (Medtronic) cautery and blunt dissection. Neurosurgical team members continued exposure and identification of the brachial plexus in the neck. The upper and middle trunks of the brachial plexus were identified posterior to the anterior scalene and secured with vessel loops. The omohyoid muscle was identified and divided. The mass was able to be elevated off the spinal vertebrae deeply and all circumferential soft-tissue attachments.
At this point, a descending limb of the incision between the middle and lateral thirds of the clavicle was created and extended caudally along the deltopectoral groove to allow for dissection to continue infraclavicularly [
After maximal safe dissection from the clavicular incisions, our thoracic surgery colleagues made an incision into the axilla for eventual mobilization of the thoracic component of the tumor. Neurosurgery identified the ongoing segments of the brachial plexus, including the terminal branches [
The curvilinear cervical incision was then extended posteriorly between the spinous processes and the medial border of the scapula to expose the trapezius muscle [
After copious irrigation and hemostasis confirmation, a Jackson Pratt drain and a blake drain were placed in the cervical portion of the dissection bed and anterior-inferior aspect of the dissection bed, respectively. The trapezius was closed with running suture. The platysma was closed in a running manner after several stitches were used to approximate supraclavicular fat over the proximal portions of the brachial plexus. The axillary incision was closed in layers with care to approximate both muscle and fat over the exposed brachial plexus to separate it from the overlying skin incision. The skin at all sites was closed in multiple layers.
Postoperatively, the patient had pain limited right upper extremity weakness which, however, had intact intrinsic hand function, palpable radial and ulnar pulses, and intact sensation. On histological examination, much of the tumor had morphology of an undifferentiated pleomorphic sarcoma. However, there were several foci of myxoid stroma with associated curvilinear vasculature. These features, in conjunction with the tumor’s infiltrative growth pattern, support the diagnosis of a high-grade MFS [
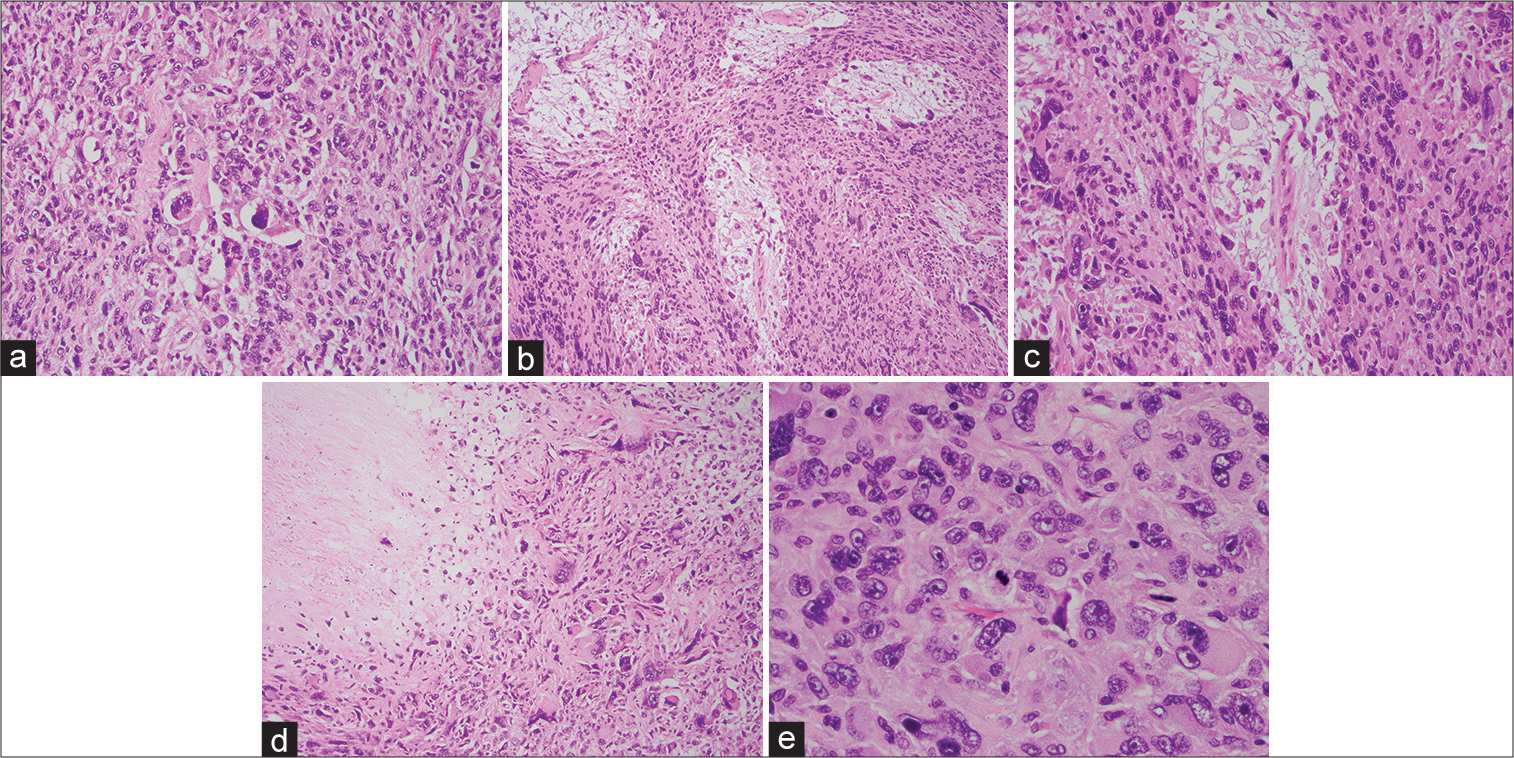
Figure 4:
Tumor histology (a) The tumor has high cellularity and severe cytologic atypia (hematoxylin and eosin, ×200 magnification). (b and c) The tumor also had focal areas with loose myxoid stroma and associated delicate vessels (hematoxylin and eosin, ×100 and ×200 magnification. (d) Necrosis was also present (upper left of image; hematoxylin and eosin, ×100 magnification). (e) Mitotic figures were also present (center of image; hematoxylin and eosin, ×400 magnification).
At his 3-week postoperative visit, the patient had pain related right-sided 4/5 deltoid weakness and resting asymmetry with the right shoulder lower than the left. However, he had 5/5 strength everywhere distally and is working with physical therapy outpatient to improve mobility.
DISCUSSION
MFS is one of the most common soft-tissue masses arising in older adults. Due to MFS’s propensity for recurrence and distant metastasis, accurate diagnosis, surgical management, and proper adjuvant therapies are crucial to patient outcomes. Preoperative staging and imaging with computed tomography (CT), ultrasound, and MRI can assist with determining the anatomical complexity of the mass. The optimum strategy for treating MFS is surgical resection with margins.[
The clinical course of MFS can vary significantly, and they are often initially asymptomatic. Our patient presented with a painless subcutaneous palpable mass of his neck and chest wall which led to further workup. With lower-grade masses, patients typically experience expansile growth, whereas higher-grade tumors often cause invasion and compression of key surrounding structures, such as airway structures in the neck and brachial plexus structures in the axilla. In our case, key features of higher-grade MFS included the mass’s infiltrative growth pattern, its association with and abutment of neurovascular structures, and its histopathological features including high cellularity, severe nuclear atypia, and tumor necrosis. The tumor in this case demonstrated adjacency, without disruption, of neurological function of the brachial plexus.
Multiple studies have demonstrated that extent of resection and tumor size is directly proportional to patient outcomes. Zumarraga et al. examined 75 patients with appendicular MFS and reported that overall survival was correlated with positive surgical margins, local recurrence, and distant metastasis.[
Adjuvant therapies for MFS are appealing due to their ability to prevent recurrence. While data are limited, multiple retrospective studies have shown an association of radiation therapy and decreased rate of recurrence.[
CONCLUSION
We present a case on the resection of a large MFS involving the neck, axilla, and chest wall through a combined team of thoracic surgery, neurosurgery, and otolaryngology-head and neck surgery. Although rare, tumors involving the brachial plexus require adequate anatomical knowledge and complex surgical planning. The anatomical complexity and rarity of tumors involving the brachial plexus impose many challenges onto surgeons. Multidisciplinary teams, each with their own expertise, allow for safe and maximal resection. This case combines four common approaches to the brachial plexus for one giant tumor resection.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and partnership
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Beane JD, Yang JC, White D, Steinberg SM, Rosenberg SA, Rudloff U. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol. 2014. 21: 2484-9
2. Callegaro D, Miceli R, Bonvalot S, Ferguson P, Strauss DC, Levy A. Impact of perioperative chemotherapy and radiotherapy in patients with primary extremity soft tissue sarcoma: Retrospective analysis across major histological subtypes and major reference centres. Eur J Cancer. 2018. 105: 19-27
3. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018. 29: iv51-67
4. Kaya M, Wada T, Nagoya S, Sasaki M, Matsumura T, Yamaguchi T. MRI and histological evaluation of the infiltrative growth pattern of myxofibrosarcoma. Skeletal Radiol. 2008. 37: 1085-90
5. Lin CN, Chou SC, Li CF, Tsai KB, Chen WC, Hsiung CY. Prognostic factors of myxofibrosarcomas: Implications of margin status, tumor necrosis, and mitotic rate on survival. J Surg Oncol. 2006. 93: 294-303
6. Look Hong NJ, Hornicek FJ, Raskin KA, Yoon SS, Szymonifka J, Yeap B. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann Surg Oncol. 2013. 20: 80-6
7. Mansoor A, White CR. Myxofibrosarcoma presenting in the skin: Clinicopathological features and differential diagnosis with cutaneous myxoid neoplasms. Am J Dermatopathol. 2003. 25: 281-6
8. Mentzel T, Calonje E, Wadden C, Camplejohn RS, Beham A, Smith MA. Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996. 20: 391-405
9. Merck C, Angervall L, Kindblom LG, Oden A. Myxofibrosarcoma. A malignant soft tissue tumor of fibroblastichistiocytic origin. A clinicopathologic and prognostic study of 110 cases using multivariate analysis. Acta Pathol Microbiol Immunol Scand Suppl 1983. 282: 1-40
10. Mutter RW, Singer S, Zhang Z, Brennan MF, Alektiar KM. The enigma of myxofibrosarcoma of the extremity. Cancer. 2012. 118: 518-27
11. Roland CL, Wang WL, Lazar AJ, Torres KE. Myxofibrosarcoma. Surg Oncol Clin N Am. 2016. 25: 775-88
12. Sanfilippo R, Miceli R, Grosso F, Fiore M, Puma E, Pennacchioli E. Myxofibrosarcoma: Prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2011. 18: 720-5
13. Waters B, Panicek DM, Lefkowitz RA, Antonescu CR, Healey JH, Athanasian EA. Low-grade myxofibrosarcoma: CT and MRI patterns in recurrent disease. AJR Am J Roentgenol. 2007. 188: W193-8
14. Willems SM, Debiec-Rychter M, Szuhai K, Hogendoorn PC, Sciot R. Local recurrence of myxofibrosarcoma is associated with increase in tumour grade and cytogenetic aberrations, suggesting a multistep tumour progression model. Mod Pathol. 2006. 19: 407-16
15. Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998. 16: 197-203
16. Zumarraga JP, Batista FA, Baptista AM, Caiero MT, Martino LP, de Camargo OP. prognostic factors in patients with appendicular myxofibrosarcoma. Acta Ortop Bras. 2018. 26: 320-4