- Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
- Department of Radiology, Medical College of Wisconsin, Milwaukee, WI, USA
Correspondence Address:
Ha Son Nguyen
Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
DOI:10.4103/2152-7806.164696
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nguyen HS, Doan N, Eckardt G, Gelsomino M, Shabani S, Brown WD, Mueller W, Pollock G. A completely thrombosed, nongiant middle cerebral artery aneurysm mimicking an intra-axial neoplasm. Surg Neurol Int 07-Sep-2015;6:146
How to cite this URL: Nguyen HS, Doan N, Eckardt G, Gelsomino M, Shabani S, Brown WD, Mueller W, Pollock G. A completely thrombosed, nongiant middle cerebral artery aneurysm mimicking an intra-axial neoplasm. Surg Neurol Int 07-Sep-2015;6:146. Available from: http://surgicalneurologyint.com/surgicalint_articles/a-completely-thrombosed-nongiant-middle-cerebral-artery/
Abstract
Background:Few reports exist regarding thrombosed aneurysms where the initial work up was concerning for a neoplasm. To date, no published reports exist regarding a nongiant thrombosed middle cerebral artery aneurysm, where the primary workup and treatment plan was directed toward a preliminary diagnosis of intra-axial neoplasm.
Case Description:We report a 43-year-old female who presented with a generalized tonic-clonic seizure attributed to a lesion along the right superior temporal gyrus. The lesion enhanced on initial magnetic resonance imaging (MRI) of the brain, as well as on follow-up MRI. Subsequent vascular studies and metastatic work up were negative. A craniotomy with image guidance was performed and an intraoperative diagnosis was made of a thrombosed aneurysm along a branch of the middle cerebral artery. The aneurysm was trapped and resected as there was no significant flow from the branch as seen on the prior cerebral angiogram. The patient had an uneventful postoperative course.
Conclusion:Completely thrombosed, nongiant aneurysms can mimic an intra-axial neoplasm. Typical imaging features for thrombosed aneurysms may be missed, especially if the aneurysms are small, where imaging characteristics of the intraluminal contents is more difficult to appreciate. Although imaging may be consistent with a neoplastic lesion, there should be suspicion for a potential underlying aneurysm.
Keywords: Complete thrombosis, middle cerebral artery, neoplasm, seizures, thrombosed cerebral aneurysm
INTRODUCTION
A “thrombosed aneurysm” is an aneurysm that exhibits a dense, structured, intraluminal thrombus, which is diagnosed based on preoperative imaging (computed tomography [CT], magnetic resonance imaging [MRI], and angiography) and perioperative findings. Thrombosed aneurysms have been classified into six types: Concentric, eccentric, lobulated, canalized, coiled, and complete.[
There have been rare reports where completely thrombosed aneurysms have imitated neoplasms based on initial presentation and imaging.[
CASE PRESENTATION
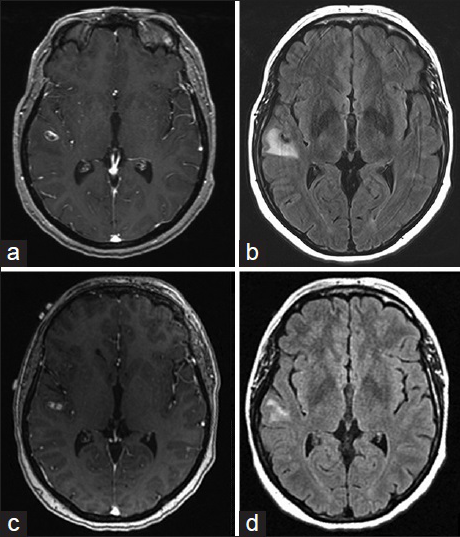
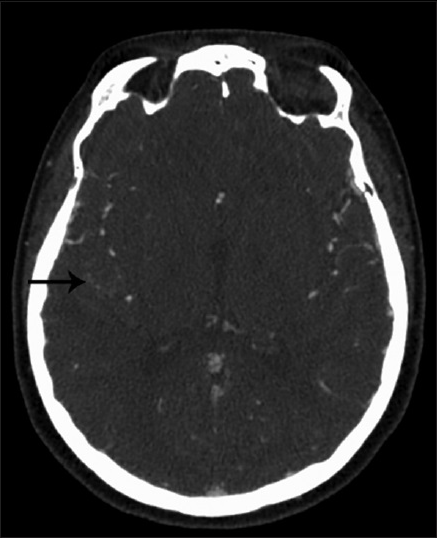
A 43-year-old female, with history of supraventricular tachycardia status postablation, hypertension, venous insufficiency, and irritable bowel syndrome, presented to the Emergency Department (ED) after a 1-min episode of generalized tonic-clonic seizure. Basic labs were normal. Given a normal neurological exam and lack of further symptoms or complaints during her ED visit, the patient was sent home for outpatient follow-up. At 2 weeks from initial presentation, a routine electroencephalogram demonstrated no findings. At 3 weeks from initial presentation, a MRI brain demonstrated an 11 mm peripherally enhancing intra-axial lesion along the right superior temporal gyrus with blood products and vasogenic edema [Figure
Due to persistent enhancement and surrounding FLAIR signal, there was a significant concern for a neoplastic process, although an inflammatory process could not be ruled out. Patient underwent a right temporal craniotomy with plans for mass excision. Dissection was taken through the superior temporal gyrus down to the level of the lesion. Some discolored brain was encountered, which was sent for permanent section. The lesion was hard and associated with sylvian arteries, consistent with a thrombosed aneurysm. There were 2 distal vessels exiting the aneurysm, which were draped over the surface of the aneurysm; these were freed from the aneurysm dome. It was felt that they were not supplying the distal territory, but emanated from the pial collaterals from the posterior circulation. After review of the preoperative cerebral angiogram, it was felt safe that these vessels could be sacrificed as there was no evidence of significant antegrade flow supplying distal territory. Any other vessels underneath were then dissected free. The proximal portion of the aneurysm and inflow vessel were then identified; the aneurysm was fusiform in appearance and was filled with significant, multiple layers of thrombus. There was a small vessel coming off at the origin of the aneurysm. At this time, a 7 mm straight permanent clip was placed across the proximal inflow of the aneurysm, taking care to preserve the small vessel at the origin. A second 7 mm straight clip was then stacked upon the first one to ensure closure of the vessel. Using microscissors and an arachnoid knife, the aneurysm was then resected, cutting the proximal portion away from the permanent aneurysm clips, and then removing the distal portion of the aneurysm. The two remaining temporary clips were then replaced with permanent straight 4 mm clips. The vessel distal to the 2 small feeders that came off the aneurysm remained pink and pulsatile and seemed to have adequate retrograde flow at this point. Subsequently, hemostasis was obtained and the wound was closed accordingly. The patient woke up neurologically intact. Final pathology confirmed a thrombosed aneurysm.
DISCUSSION
The cause of seizure activity in our patient is not entirely clear. The aneurysm may have induced local irritation without hemorrhage; a small mass invaginating the cortex could conceivably do so through minimal swelling and ischemia. On the other hand, the aneurysm may have exhibited a mild subarachnoid hemorrhage (SAH). MRI brain T2 FLAIR/gradient-recalled echo sequences are usually sufficient to diagnose SAH.[
For patients who demonstrate spontaneous SAH, initial CT angiography and digital subtraction angiography (DSA) may fail to diagnose the source of bleeding for up to 15–30% of cases.[
CT features suggestive of aneurysmal thrombosis include varied high density within the lumen, the presence of the “target sign,” peripheral rim enhancement, and curvilinear mural calcification.[
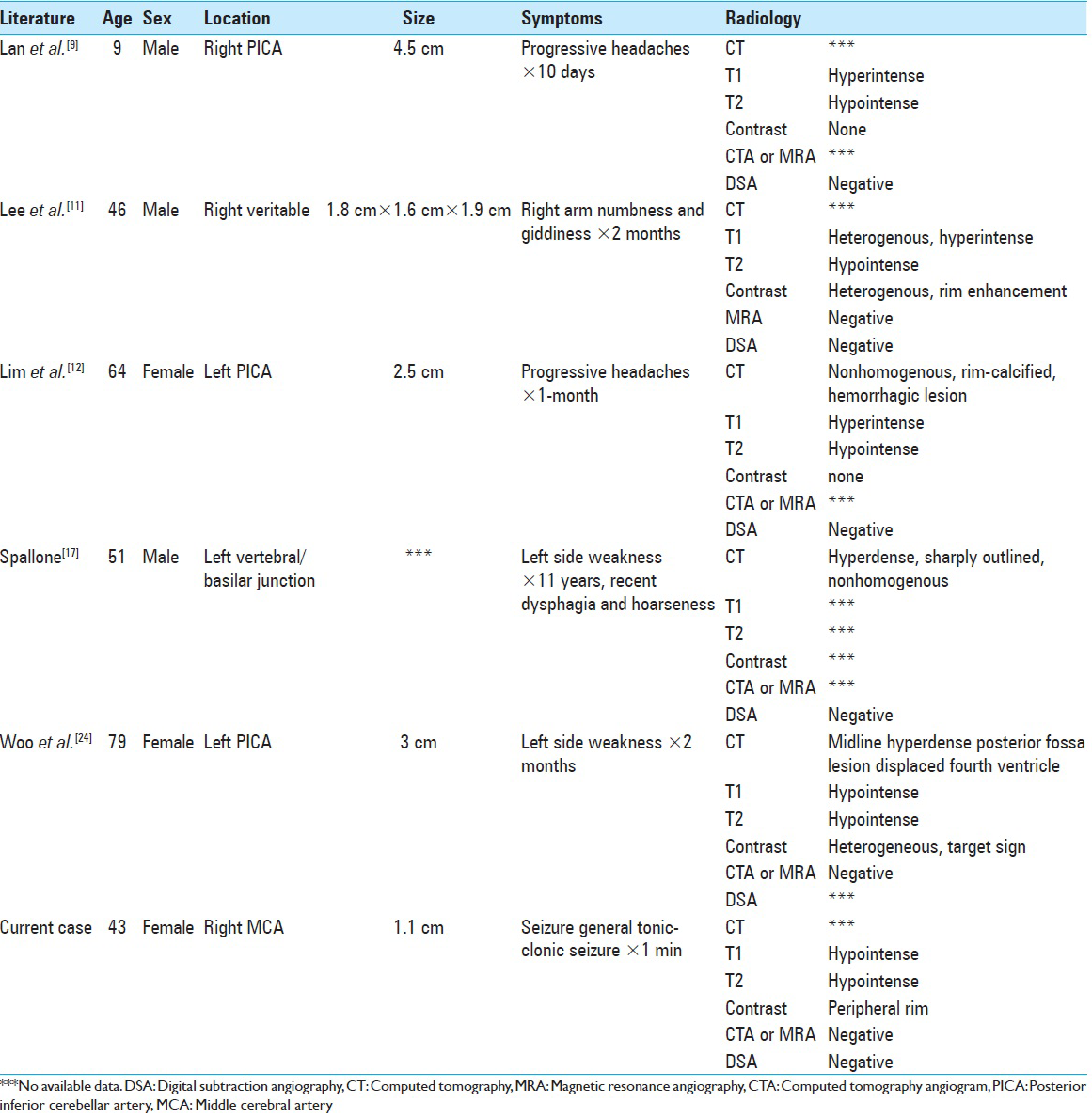
A literature review concerning completely thrombosed aneurysms that mimicked neoplasms revealed a handful of case reports [
CONCLUSION
Completely thrombosed, nongiant aneurysms may mimic an intra-axial neoplasm. Typical imaging features for thrombosed aneurysms may be missed, especially if the aneurysms are small, where resolution of intraluminal contents is more difficult to appreciate. Peripheral rim enhancement, which has been attributed to thrombosed aneurysms, could also strengthen concerns for a neoplasm, especially if peri-lesional FLAIR signal is present. Although imaging may be consistent for a neoplasm, there should be suspicion for a potential underlying aneurysm, which would alter surgical management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bakker NA, Groen RJ, Foumani M, Uyttenboogaart M, Eshghi OS, Metzemaekers JD. Repeat digital subtraction angiography after a negative baseline assessment in nonperimesencephalic subarachnoid hemorrhage: A pooled data meta-analysis. J Neurosurg. 2014. 120: 99-103
2. Calviere L, Viguier A, Da Silva NA, Cognard C, Larrue V. Unruptured intracranial aneurysm as a cause of cerebral ischemia. Clin Neurol Neurosurg. 2011. 113: 28-33
3. da Rocha AJ, da Silva CJ, Gama HP, Baccin CE, Braga FT, Cesare Fde A, Veiga JC. Comparison of magnetic resonance imaging sequences with computed tomography to detect low-grade subarachnoid hemorrhage: Role of fluid-attenuated inversion recovery sequence. J Comput Assist Tomogr. 2006. 30: 295-303
4. Dashti R, Rinne J, Hernesniemi J, Niemelä M, Kivipelto L, Lehecka M. Microneurosurgical management of proximal middle cerebral artery aneurysms. Surg Neurol. 2007. 67: 6-14
5. De Luca GP, Volpin L, Colombo F, Fornezza U, Pinna V. Spontaneous healing of cerebral aneurysm at the beginning of left posterior inferior cerebellar artery (PICA). Case report. J Neurosurg Sci. 1998. 42: 115-8
6. Edner G, Forster DM, Steiner L, Bergvall U. Spontaneous healing of intracranial aneurysms after subarachnoid hemorrhage. Case report. J Neurosurg. 1978. 48: 450-4
7. Greenberg E, Janardhan V, Katz JM, Riina H, Zimmerman R, Gobin YP. Disappearance and reappearance of a cerebral aneurysm: A case report. Surg Neurol. 2007. 67: 186-8
8. Iwanaga H, Wakai S, Ochiai C, Narita J, Inoh S, Nagai M. Ruptured cerebral aneurysms missed by initial angiographic study. Neurosurgery. 1990. 27: 45-51
9. Lan ZG, Ma L, Duan J, You C. A fully thrombosed giant posterior inferior cerebellar aneurysm mimicking an intracranial tumour in a child. Br J Neurosurg. 2012. 26: 888-90
10. Lawton MT, Quiñones-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms: Classification scheme and management strategies in 68 patients. Neurosurgery. 2005. 56: 441-54
11. Lee W, Hui F, Sitoh YY. Intravascular papillary endothelial hyperplasia in an intracranial thrombosed aneurysm: 3T magnetic resonance imaging and angiographical features. Singapore Med J. 2004. 45: 330-3
12. Lim DH, Jung S, Jung TY, Kim TS. An Unusual Case of a Thrombosed Giant Distal PICA Aneurysm Simulating a Large Cavernous Angioma. J Korean Neurosurg Soc. 2008. 43: 155-8
13. Martin AJ, Hetts SW, Dillon WP, Higashida RT, Halbach V, Dowd CF. MR imaging of partially thrombosed cerebral aneurysms: Characteristics and evolution. AJNR Am J Neuroradiol. 2011. 32: 346-51
14. Roccatagliata L, Guédin P, Condette-Auliac S, Gaillard S, Colas F, Boulin A. Partially thrombosed intracranial aneurysms: Symptoms, evolution, and therapeutic management. Acta Neurochir (Wien). 2010. 152: 2133-42
15. Ruelle A, Lasio G, Boccardo M, Gottlieb A, Severi P. Long-term prognosis of subarachnoid hemorrhages of unknown etiology. J Neurol. 1985. 232: 277-9
16. Santiago-Dieppa DR, Pannell JS, Khalessi AA. Endovascular and surgical options for ruptured middle cerebral artery aneurysms: Review of the literature. Stroke Res Treat 2014. 2014. p.
17. Spallone A. Giant, completely thrombosed intracranial aneurysm simulating tumor of the foramen magnum. Surg Neurol. 1982. 18: 372-6
18. Spetzler RF, Winestock D, Newton HT, Boldrey EB. Disappearance and reappearance of cerebral aneurysm in serial arteriograms. Case report. J Neurosurg. 1974. 41: 508-10
19. Su TM, Hsu SW, Chen WF, Lee TC, Cheng CH. Acute thrombosis and recanalization of a ruptured anterior communicating artery aneurysm. J Clin Neurosci. 2009. 16: 1077-9
20. Topcuoglu MA, Ogilvy CS, Carter BS, Buonanno FS, Koroshetz WJ, Singhal AB. Subarachnoid hemorrhage without evident cause on initial angiography studies: Diagnostic yield of subsequent angiography and other neuroimaging tests. J Neurosurg. 2003. 98: 1235-40
21. Verma RK, Kottke R, Andereggen L, Weisstanner C, Zubler C, Gralla J. Detecting subarachnoid hemorrhage: Comparison of combined FLAIR/SWI versus CT. Eur J Radiol. 2013. 82: 1539-45
22. Wei D, Jingru Z, Cungang F, Yake X, Dongliang W, Zhengmao W. Complete spontaneous thrombosis and recanalization of a ruptured posterior cerebral artery aneurysm. Turk Neurosurg. 2014. 24: 406-10
23. Whittle IR, Dorsch NW, Besser M. Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1982. 45: 1040-7
24. Woo PY, Ko NM, Chan KY. Thrombosed large distal posterior inferior cerebellar artery aneurysm mimicking an infratentorial ependymoma. Case Rep Neurol Med 2014. 2014. p.
25. Yuan MK, Lai PH, Chen JY, Hsu SS, Liang HL, Yeh LR. Detection of subarachnoid hemorrhage at acute and subacute/chronic stages: Comparison of four magnetic resonance imaging pulse sequences and computed tomography. J Chin Med Assoc. 2005. 68: 131-7