- Department of Neurosurgery, Ehime University Graduate School of Medicine, Toon, Japan
- Department of Neurosurgery, Ehime Prefectural Central Hospital, Matsuyama, Japan
- Department of Pediatric Neurosurgery, Takatsuki General Hospital, Takatsuki, Japan
- Department of Pediatrics, Ehime University Graduate School of Medicine, Toon, Japan.
Correspondence Address:
Shirabe Matsumoto, Department of Neurosurgery, Ehime University Graduate School of Medicine, Toon, Japan.
DOI:10.25259/SNI_585_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shirabe Matsumoto1, Shinji Iwata2, Atsuko Harada3, Hikaru Imon4, Toshimoto Seno2, Hideaki Watanabe1, Takeharu Kunieda1. A large growing occipital meningocele with Dandy–Walker syndrome: A case report and review of the literature. 06-Oct-2023;14:353
How to cite this URL: Shirabe Matsumoto1, Shinji Iwata2, Atsuko Harada3, Hikaru Imon4, Toshimoto Seno2, Hideaki Watanabe1, Takeharu Kunieda1. A large growing occipital meningocele with Dandy–Walker syndrome: A case report and review of the literature. 06-Oct-2023;14:353. Available from: https://surgicalneurologyint.com/surgicalint-articles/12585/
Abstract
Background: Dandy–Walker syndrome (DWS) is a well-known developmental anomaly. An occipital meningocele (OMC) is recognized as a malformation that is relatively often associated with DWS, but the association of DWS with OMC has been reported in approximately 40 cases. We present herein a rare clinical course of DWS with OMC, in which the sac was small at birth and became progressively larger.
Case Description: A 5-day-old baby boy was referred to our hospital due to OMC. He was born at 33 gestational weeks due to premature rupture of the membranes. He was diagnosed as having DWS associated with OMC. The OMC was covered with skin and its maximum diameter at birth was 3 cm. Magnetic resonance imaging showed an occipital bone defect and continuity of the fourth ventricle, posterior fossa cyst, and OMC sac. The aqueduct was patent, and no hydrocephalus was found. The OMC sac increased progressively with moderate hydrocephalus and reached 7 cm at the age of 54 days when his weight was 2508 g. A cystoperitoneal shunt and repair were performed after sinus venography by contrast computed tomography (CT). At the age of 1 year and 8 months, he had moderate developmental disabilities.
Conclusion: In most cases reported, the OMC was relatively small, and large and giant sizes were reported in only six cases. Almost all cases remained the same size as at birth and underwent surgical intervention as early as possible. It was possible to understand the relationship between the occipital bone defect and abnormal running of sinuses such as the superior sagittal sinus, torcular Herophili, and transverse sinus preoperatively from the CT venography (CTV) image. CTV may be an effective and important method for safely performing repair and shunt.
Keywords: Cyst-peritoneal shunt, Dandy–Walker syndrome, Occipital meningocele
INTRODUCTION
Dandy–Walker syndrome (DWS) is a well-recognized developmental anomaly of the brain consisting of cerebellar vermis hypoplasia or agenesis, cystic dilatation of the fourth ventricle, enlarged posterior fossa, and a high tentorium. Many congenital malformations of the central nervous system such as hydrocephalus, agenesis of the corpus callosum, cerebral grey abnormalities, heterotopias, syringomyelia, aqueductal stenosis, malformation of the inferior olivary nuclei and dendritic nuclei, and hypoplasia of the pyramidal tracts are associated with DWS.[
CASE PRESENTATION
A boy was suspected of having DWS at 31 gestational weeks on fetal ultrasonography based on the findings of a cyst in the posterior fossa. He was delivered vaginally at 33 gestational weeks due to premature rupture of the membranes. His general condition was good (Apgar score 8/9), with a birth weight of 1969 g and a head circumference of 31 cm. However, he was found to have a ventricular septal defect and pulmonary hypertension which resulted in polypnea and retracted breathing. Therefore, he was transferred to our institution 5 days after birth for further evaluation and treatment. There was no family history of any congenital malformation or neural tube defect. An occipital bone defect with a skin-covered mass, 3 cm * 3 cm * 1 cm in size, was observed. A hairless part covered with fibrous tissues was found above the mass [
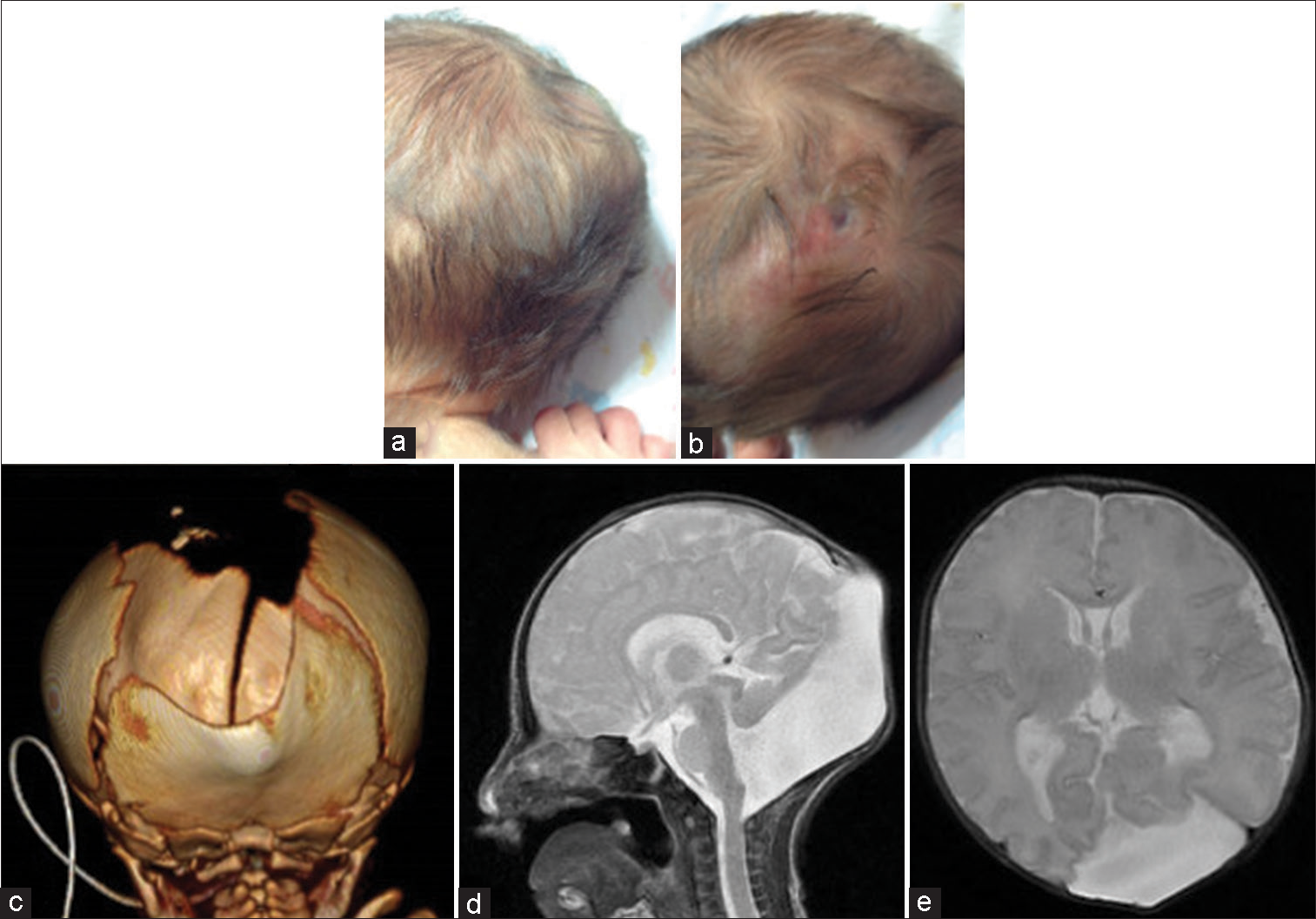
Figure 1:
Images of the patient at the age of 5 days. (a and b) Clinical photographs show the skin-covered mass of size 3 cm*3 cm*1 cm with a hairless part covered with fibrous tissues. (c) Three-dimensional computed tomography shows sagittal suture dilatation and an occipital bone defect. (d and e) On sagittal and axial magnetic resonance imaging, a large posterior fossa, vermis hypoplasia, and a posterior fossa cyst communicating with the fourth ventricle can be seen, confirming the Dandy–Walker syndrome (DWS). The cystic cerebrospinal fluid-intensity small swelling in the occipital region is a large cyst communicating with a posterior fossa cyst through a defect in the occipital bone, suggesting an occipital meningocele associated with DWS. Hydrocephalus and occlusion of the aqueduct are not found.
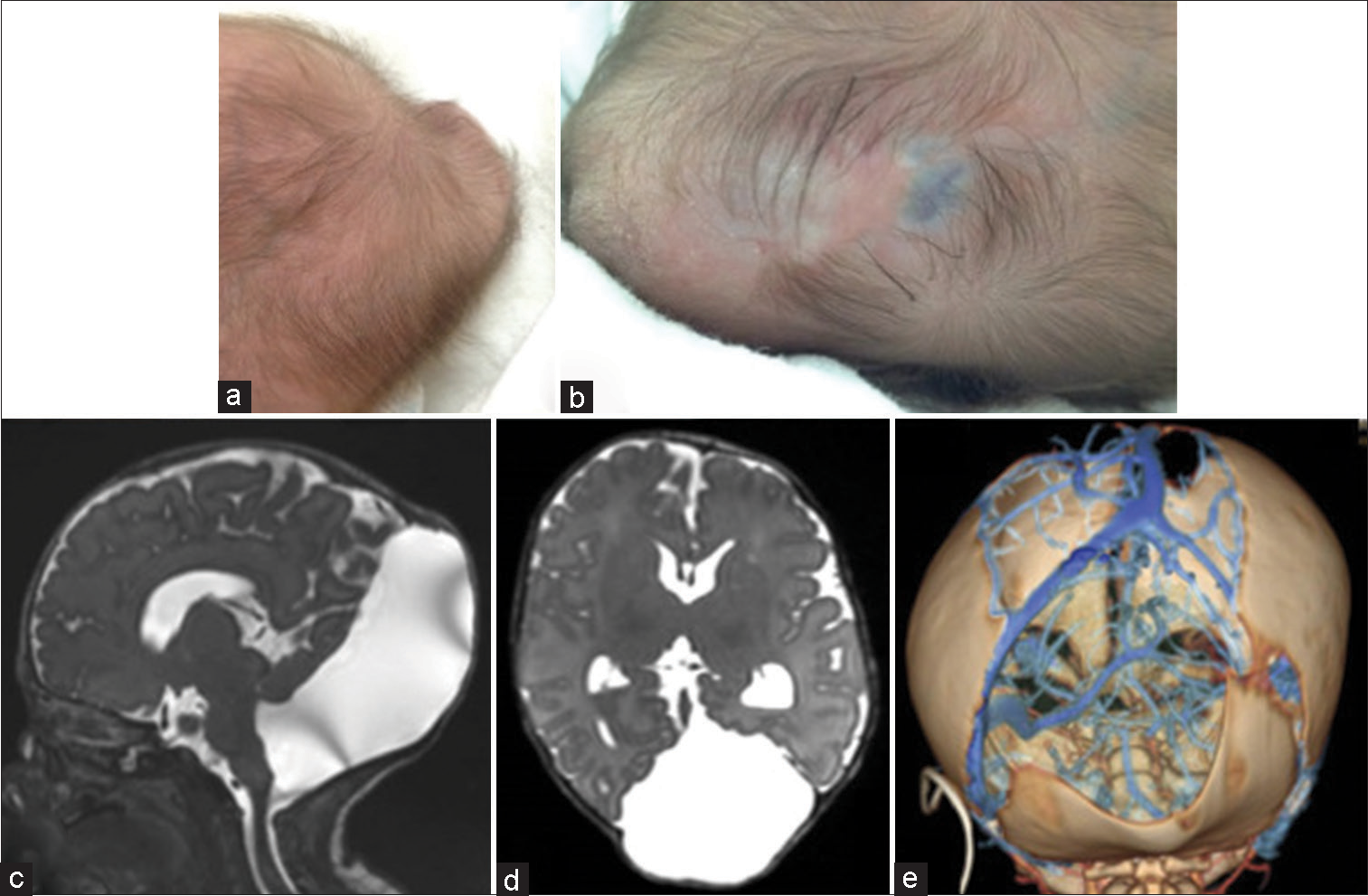
Figure 2:
Images of the patient at the age of 50 days. (a and b) Clinical photographs show that the skin-covered subcutaneous mass in the occipital region has enlarged to 7 cm*7 cm*2.5 cm. The hairless part covered with fibrous tissues is further thinned, and the inside of the dura is seen through it. (c and d) On sagittal and axial magnetic resonance imaging, an occipital meningocele communicating with a posterior fossa cyst and fourth ventricle, and mild ventricular enlargement indicating hydrocephalus are seen. (e) Three-dimensional computed tomography venography demonstrates the run of the sinuses and veins in a defective part of the bone. The left transverse sinus runs along the left edge of an occipital bone defect, but there is neither sinus nor veins in its right margin. Both the occipital bone defect and sagittal suture dilatation are enlarged.
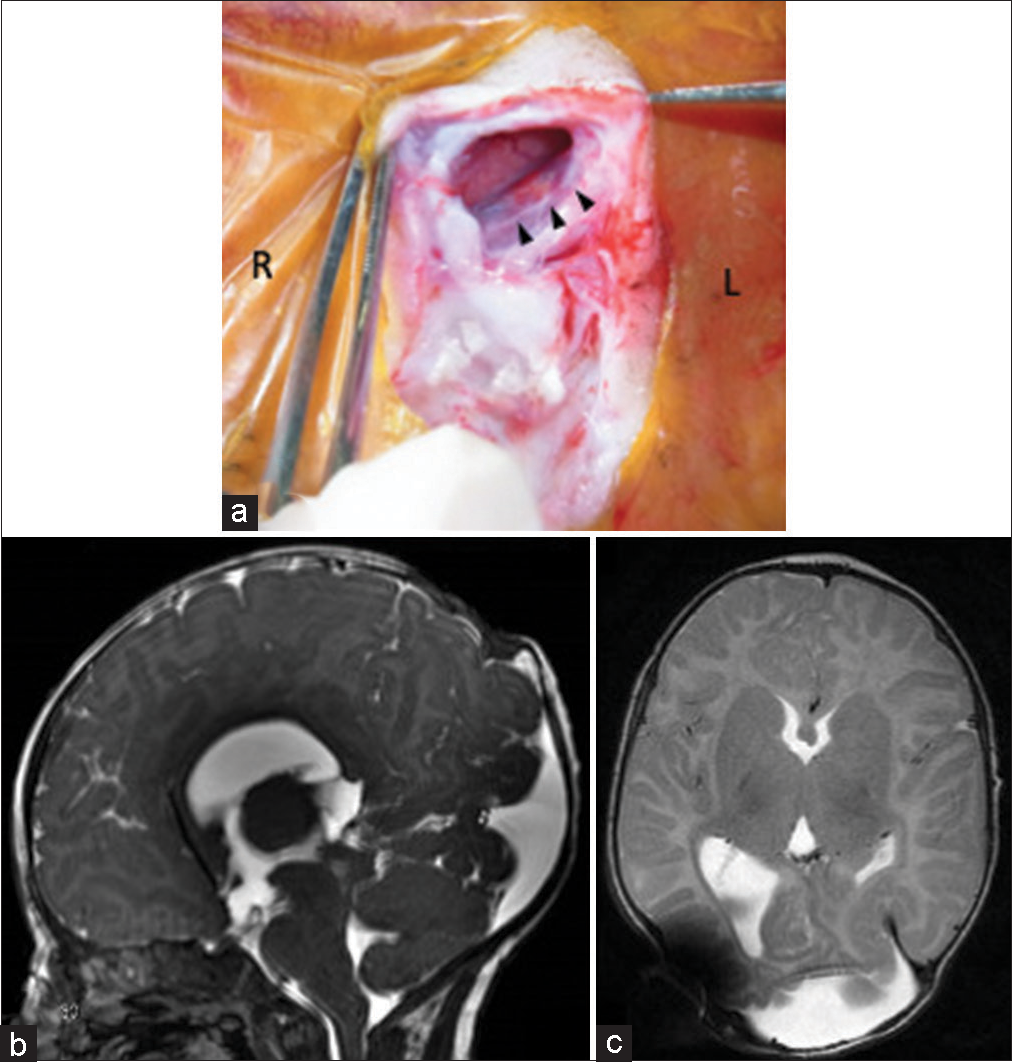
Figure 3:
(a) Intraoperative photograph shows the left transverse sinus (black arrow) running along the left edge of the meningocele sac after the dura incision. (b and c) On sagittal and axial magnetic resonance imaging 2 months after surgery, the contracted occipital meningocele and ventricles are seen.
DISCUSSION
A cranial meningocele consists of the herniation of only dura mater through a skull defect, whereas an encephalocele includes the herniation of dura mater with neural tissue.[
DWS with OMC
OMC repair and shunting
In
Bindal et al. recommended only shunt placement as the initial surgical treatment of DWS with OMC and that surgical closure of the OMC may not be necessary.[
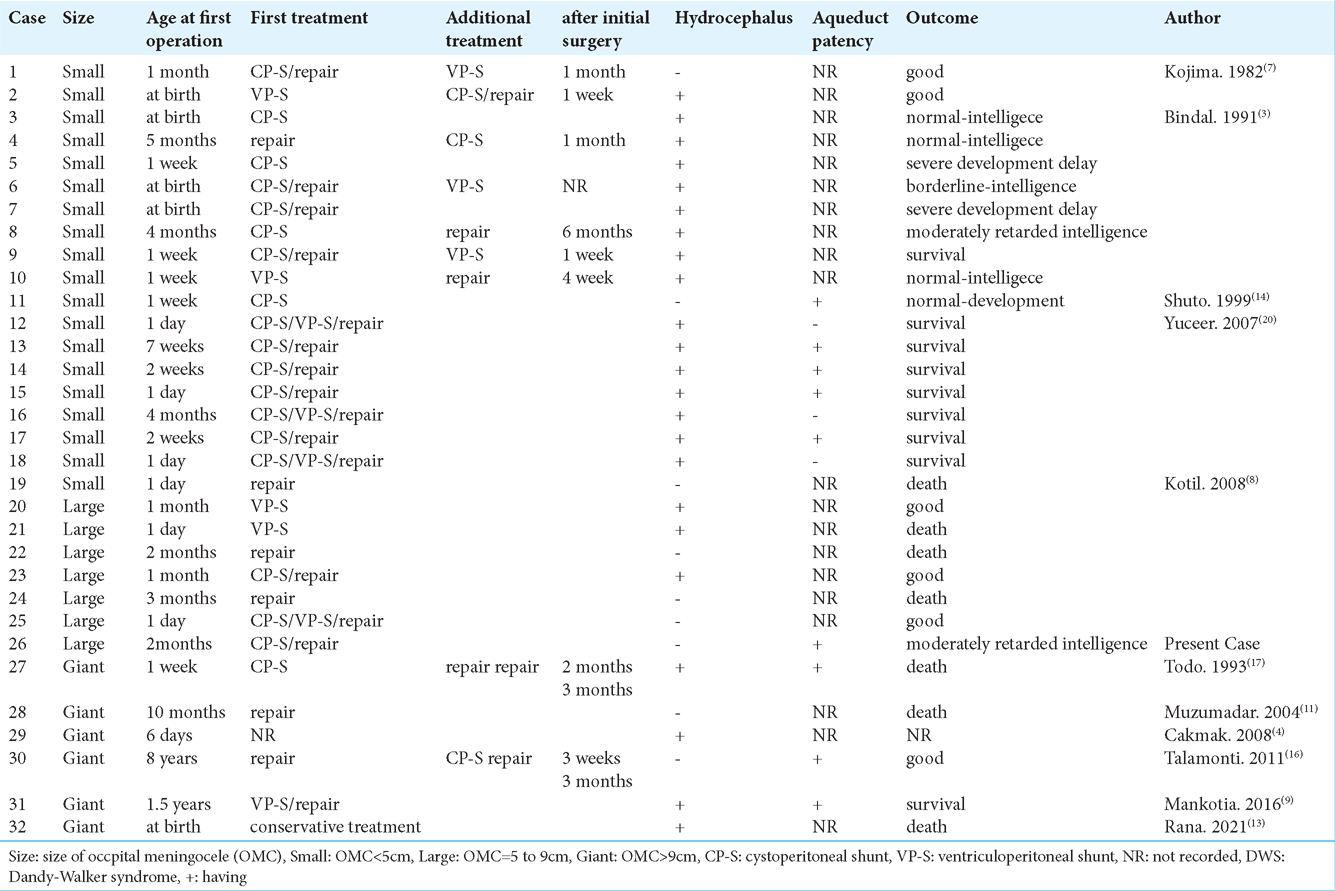
On the other hand, of the three patients with a large or giant OMC who underwent only shunt placement two patients died [
CP-S or VP-S
There is no clear guideline for deciding between CP-S and VP-S for patients having DWS with OMC. As the initial shunt placement in the cases of DWS with OMC that we found, including the present case, 15 patients underwent CPS, six patients underwent VP-S placement, and four patients underwent combined shunt surgery (CP-S plus VP-S) [
Usefulness of CTV
To safely perform shunting and repair for patients having DWS with OMS, it is necessary to understand the anatomy of the cyst. In particular, the relationship between the OMC sac and the vein or sinus is important. Verma et al. reported that magnetic resonance venography (MRV) may show a dilated venous sinus and torcula, and it aids in proper surgical planning in cases of occipital encephalocele.[
CONCLUSION
Although almost all cases remained the same size as at birth and underwent surgical intervention as early as possible, the present case of OMC that developed progressively and reaching a large size was rare. Similar to most cases, both shunt placement and repair were performed at the same time as the first treatment. Confirming significant enlargement of the OMC sac despite mild hydrocephalus, CP-S was selected, and the outcome was good. It was possible to understand the relationship between the occipital bone defect and abnormal running of the sinuses preoperatively from the CTV image. CTV may be an effective and important method for safely performing repair and shunting.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alexiou GA, Sfakianos G, Prodromou N. Dandy-Walker malformation: Analysis of 19 cases. J Child Neurol. 2010. 25: 188-91
2. Asai A, Hoffman HJ, Hendrick EB, Humphreys RP. Dandy-Walker syndrome: Experience at the hospital for sick children, Toronto. Pediatr Neurosci. 1989. 15: 66-73
3. Bindal AK, Storrs BB, McLone DG. Occipital meningoceles in patients with the Dandy-Walker syndrome. Neurosurgery. 1991. 28: 844-7
4. Cakmak A, Zeyrek D, Cekin A, Karazeybek H. Dandy-Walker syndrome together with occipital encephalocele. Minerva Pediatr. 2008. 60: 465-8
5. Hawkins JC, Hoffman HJ, Humphreys RP. Isolated fourth ventricle as a complication of ventricular shunting. J Neurosurg. 1978. 49: 910-3
6. Jha VC, Kumar R, Srivastav AK, Mehrotra A, Sahu RN. A case series of 12 patients with incidental asymptomatic Dandy-Walker syndrome and management. Childs Nerv Syst. 2012. 28: 861-7
7. Kojima T, Waga S, Shimizu T, Sakakura T. Dandy-Walker cyst associated with occipital meningocele. Surg Neurol. 1982. 17: 52-6
8. Kotil K, Kilinc B, Bilge T. Diagnosis and management of large occipitocervical cephaloceles: A 10-year experience. Pediatr Neurosurg. 2008. 44: 193-8
9. Mankotia DS, Satyarthee GD, Singh B, Sharma BS. A rare case of giant occipital meningocele with Dandy Walker Syndrome: Can it grow bigger than this?. J Pediatr Neurosci. 2016. 11: 344-7
10. Mohanty A, Biswas A, Satish S, Praharaj SS, Sastry KV. Treatment options for Dandy-Walker malformation. J Neurosurg. 2006. 105: 348-56
11. Muzumadar DP, Goel A. Giant occipital meningocele as a present feature of Dandy-Walker syndrome. Indian Pediatr. 2004. 41: 863-4
12. Pascual-Castroviejo I, Velez A, Pascual-Pascual SI, Roche MC, Villarejo F. Dandy-Walker malformation: Analysis of 38 cases. Childs Nerv Syst. 1991. 7: 88-97
13. Rana A, Chawla D. Rare Association of Dandy-Walker malformation with a giant occipital meningocele. J Obstet Gynaecol Can. 2021. 43: 795
14. Shuto T, Sekido K, Ohtsubo Y, Saida A, Yamamoto I. Dandy-Walker syndrome associated with occipital meningocele and spinal lipoma--case report. Neurol Med Chir (Tokyo). 1999. 39: 544-7
15. Spennato P, Mirone G, Nastro A, Buonocore MC, Ruggiero C, Trischitta V. Hydrocephalus in Dandy-Walker malformation. Childs Nerv Syst. 2011. 27: 1665-81
16. Talamonti G, Picano M, Debernardi A, Bolzon M, Teruzzi M, D’Aliberti G. Giant occipital meningocele in an 8-year-old child with Dandy-Walker malformation. Childs Nerv Syst. 2011. 27: 167-74
17. Todo T, Usui M, Araki F. Dandy-Walker syndrome forming a giant occipital meningocele--case report. Neurol Med Chir (Tokyo). 1993. 33: 845-50
18. Verma SK, Satyarthee GD, Singh PK, Sharma BS. Torcular occipital encephalocele in infant: Report of two cases and review of literature. J Pediatr Neurosci. 2013. 8: 207-9
19. Yamashita M, Daizo H, Shimada K. Three-dimensional computed tomography venography as a guide for cranioplasty in parietal cephalocele. J Craniofac Surg. 2014. 25: 224-5
20. Yüceer N, Mertol T, Arda N. Surgical treatment of 13 pediatric patients with Dandy-Walker syndrome. Pediatr Neurosurg. 2007. 43: 358-63